Acta medica Lituanica ISSN 1392-0138 eISSN 2029-4174
2025. Vol. 32. No 2. Online ahead of print DOI: https://doi.org/10.15388/Amed.2025.32.2.14
Rassul Zhumagaliyev*
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: 777rassul777@gmail.com
Yerlan Orazymbetov
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania; National Scientific Medical Center, Astana, Kazakhstan
E-mail: doctoryerlan@gmail.com
ORCID ID https://orcid.org/0009-0008-0844-7268
Serik Aitaliyev
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania; Faculty of Medicine and Health Care, Al-Farabi Kazakh National University, Almaty, Kazakhstan
E-mail: aitaliyev.serik@gmail.com
ORCID ID https://orcid.org/0000-0002-3014-9100
Sidar Arslan
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: sidar.arslan@stud.lsmu.lt
Tadas Lenkutis
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: tadas.lenkutis@lsmu.lt
Adakrius Siudikas
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: adakrius.siudikas@lsmu.lt
Rimantas Benetis
Department of Cardiac, Thoracic and Vascular Surgery, Hospital of Lithuanian University of Health Sciences, Kauno Klinikos, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: rimantas.benetis@lsmu.lt
Abstract. Background: Giant descending thoracic aortic aneurysm (GDTAA) is a rare vascular disease characterized by an aortic diameter exceeding 10 cm. GDTAA carries a significant risk of rupture and mortality and requires timely diagnosis and intervention. Despite the clinical severity of the disease, the literature on GDTAA remains sparse, particularly in cases with extreme aneurysmal dilatation.
Case Presentation: We present the case of a 68-year-old man with a GDTAA of 14.08 × 10.04 cm, one of the largest ever reported. The patient initially presented with recurrent syncope, chronic cough and fatigue. Imaging studies, including Computed Tomography (CT) angiography, revealed a massive aneurysmal dilatation in the distal post-arch segment of the descending aorta with compression of the trachea and bronchi. The patient underwent a successful open surgical repair with a Dacron graft and simultaneous Coronary Artery Bypass Grafting (CABG). Postoperative complications included respiratory acidosis, emphysema and transient haemodynamic instability, which were effectively treated. The patient was discharged in a stable condition on the tenth postoperative day.
Conclusion: This case highlights the importance of early recognition and surgical intervention in GDTAA in order to prevent catastrophic consequences. Comprehensive preoperative evaluation, careful surgical planning and attentive postoperative care are essential for optimal recovery. Our results emphasise the importance of modern imaging techniques for accurate anatomical assessment and risk stratification in patients with extreme aneurysm growth. Further research is needed to establish standardised protocols for the treatment of GDTAA.
Keywords: giant descending thoracic aortic aneurysm (GDTAA), aortic dilation, surgical repair.
Santrauka. Įvadas: Didelė nusileidžiančiosios krūtinės aortos aneurizma (NKAA) yra reta kraujagyslių patologija, kai aortos skersmuo viršija 10 cm. Ši aneurizma pasižymi didele plyšimo ir mirtingumo rizika, todėl laiku atlikta diagnostika ir chirurginis gydymas yra gyvybiškai svarbūs. Literatūroje didelė NKAA aprašoma retai, ypač tokie atvejai, kai aneurizma siekia 10 cm ir daugiau.
Atvejo pristatymas: 68 metų vyrui diagnozuota didelė NKAA, ji buvo 14,08×10,04 cm – tai vienas iš retų literatūroje aprašytų atvejų. Pacientas kreipėsi į gydymo įstaigą dėl pasikartojančių sinkopių, lėtinio kosulio ir nuovargio. Vaizdiniai tyrimai, įskaitant kompiuterinę tomografiją (KT) su angiografija, parodė esant didžiulę aneurizmą distaliojoje aortos lanko dalyje, sukėlusią trachėjos ir bronchų spaudimą. Pacientui sėkmingai atlikta atvira chirurginė rekonstrukcija, naudojant Dacron protezą, kartu atliktas ir vainikinių arterijų šuntavimas (AKŠ). Pooperacines komplikacijas – respiracinę acidozę, emfizemą ir laikiną hemodinaminį nestabilumą pavyko sėkmingai suvaldyti. Pacientas dešimtą pooperacinę parą išrašytas stabilios būklės.
Išvados: Šis atvejis pabrėžia ankstyvosios didelės NKAA diagnostikos ir chirurginio gydymo svarbą, siekiant išvengti sunkių padarinių. Išsamus priešoperacinis įvertinimas, kruopštus chirurginis planavimas ir atidi pooperacinė priežiūra yra būtini siekiant optimalių rezultatų. Ypač svarbi pažangių vaizdinių tyrimų metodų reikšmė tiksliai pacientų, kuriems nustatyta didelė aortos aneurizma, anatomijos analizei ir rizikos stratifikacijai. Reikia tolesnių tyrimų, siekiant nustatyti standartizuotus didelių NKAA gydymo protokolus.
Raktažodžiai: Didelė nusileidžiančiosios krūtinės aortos aneurizma (NKAA), aortos išsiplėtimas, chirurginis gydymas
_______
* Corresponding author
Received: 09/06/2025. Revised: 31/08/2025. Accepted: 20/09/2025
Copyright © 2025 Rassul Zhumagaliyev, Yerlan Orazymbetov, Serik Aitaliyev, Sidar Arslan, Tadas Lenkutis, Adakrius Siudikas, Rimantas Benetis. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Giant descending thoracic aortic aneurysm (GDTAA) is an extremely rare vascular disease with limited representation in the existing literature due to its rarity [1]. Although various studies have provided detailed definitions and classifications, GDTAA is typically characterised when the maximum transverse diameter of the aorta exceeds 10 cm, regardless of sex. While most patients with symptomatic aneurysms are diagnosed before they reach such significant proportions, asymptomatic individuals can reach extreme proportions without complications [2]. Although the reported incidence of GDTAA varies geographically, the overall average prevalence of thoracic aortic aneurysms is approximately 10 per 100,000 individuals [3].
The diagnosis of GDTAA requires a thorough differential diagnostic approach to differentiate it from conditions such as Acute Coronary Syndrome (ACS), valvular heart disease or typical heart failure symptoms, all of which may have overlapping clinical features that are influenced by the size of the aneurysm [4]. Due to the chronic nature and variable presentation of the disease, the characteristics of pain during acute episodes are often considered unreliable for distinguishing between ACS and thoracic aortic aneurysm [5, 6]. In this context, a comprehensive evaluation of the patient’s medical history plays a crucial role in the selection of appropriate diagnostic tools, such as Computed Tomography (CT), especially in emergency situations where timely diagnosis is crucial to reduce mortality. For example, a history of myocardial infarction and diabetes are strongly associated with ACS.
Patients with descending thoracic aortic aneurysms less than 6 cm in diameter may be considered for surgical intervention if they have an underlying genetic disorder (Marfan, Ehlers-Danlos, Loeys-Dietz or Turner syndrome) [7]. Without this genetic predisposition, usually recommended conservative treatment. Elective surgical repair is generally indicated if the aneurysm has a transverse diameter of more than 6.5 cm. If the aneurysm is expanding at a rate of more than 1 cm per year, earlier surgical intervention may be warranted, even for smaller diameters, so that to reduce the risk of rupture or dissection [8].
The clinical presentation of giant thoracic aortic aneurysms is primarily due to the mass effect of the aneurysm and less to heart failure or aortic regurgitation. As the aneurysm enlarges, it can compress the surrounding anatomical structures, including the left recurrent laryngeal, phrenic, and vagus nerves, resulting in persistent symptoms such as nausea and cough that are unresponsive to medication. In addition, significant airway compression can mimic features of obstructive lung disease, manifesting as dyspnoea, wheezing, and chest discomfort [9].
A rare but life-threatening complication of GDTAA is the formation of aortoesophageal or aortotracheal fistulas. These fistulas are observed more frequently in large aneurysms than in smaller aneurysms. Their formation may be related to underlying pathological conditions such as oesophageal carcinoma, mediastinal tuberculosis, trauma or postoperative complications that lead to abnormal connections between the aorta and adjacent structures such as the oesophagus. Without surgical intervention, the prognosis is consistently fatal, with a reported mortality rate of 100%, while surgical repair is associated with a high perioperative mortality of 30 to 80% [10]. In addition, in patients with chronic leukaemia, leukaemic infiltration of the mediastinal tissue surrounding a giant aneurysm can complicate the condition, potentially increasing the risk of severe vascular complications if not addressed promptly [11].
The present clinical case describes a patient with GDTAA, distinguished by one of the largest recorded transverse diameters at the time of diagnosis, accompanied by an elevated initial risk of rupture and mortality.
A 68-year-old male patient with a medical history of stage 2 hypertension and dyslipidemia presented to the cardiology clinic due to recurrent episodes of syncope. Upon admission, the patient also reported symptoms of cough and fatigue. The patient’s medical history was notable for chronic B-cell lymphocytic leukemia, which was in remission at the time of admission to the cardiac surgery department. Surgical history included previous operations for appendicitis and gastric perforation. The patient had a 45-year history of smoking, limited to 2–3 cigarettes per day. Social history was unremarkable, with no family history of aortic aneurysms or genetic disorders to be noted. Family history included hypertension in first-degree relatives but no documented vascular diseases.
Physical examination findings were unremarkable, with no audible bruits, masses, or neurological deficits. Vital signs on admission were as follows: blood pressure 150/90 mmHg, heart rate 60 bpm, respiratory rate 18/min, oxygen saturation 96% on room air.

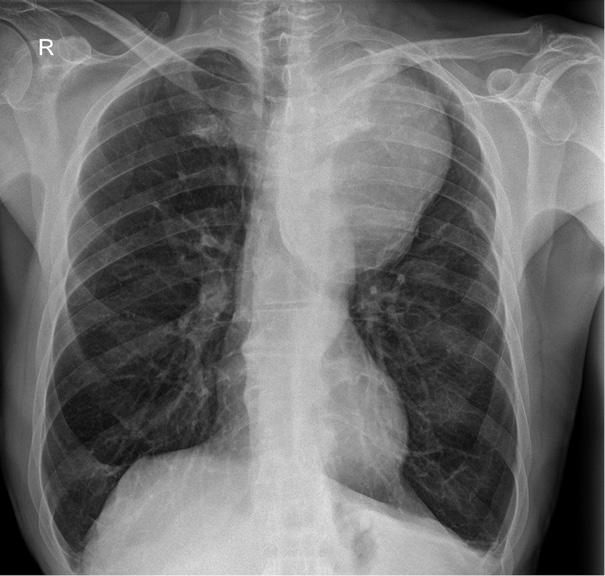
The detection of a mediastinal mass prompted differential diagnostic considerations, primarily focusing on two major possibilities: (1) mediastinal tumor formation associated with lymphocytic infiltration due to leukemia or other oncological masses, and (2) a giant vascular aneurysm. To delineate the etiology, a two-dimensional CT scan of the thoracic aorta was performed, as shown in Figure 2, revealing a large aneurysm in the distal post-arch segment of the descending aorta. The observed mass corresponds to a giant aneurysmal dilation.
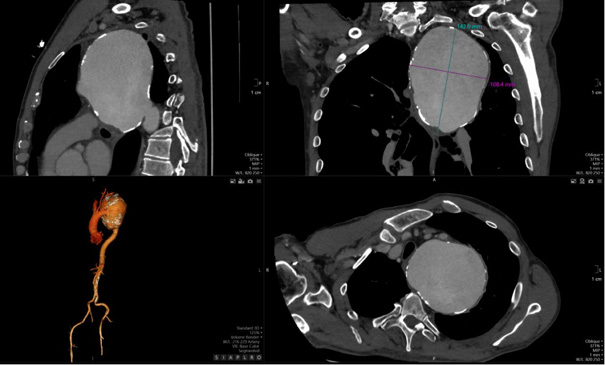
The aneurysm measured 14.08 × 10.04 cm in its maximum transverse diameters. No chronic thrombus was identified within the tunica media, nor was there any evidence of dissection. The proximal ascending aorta measured 38 mm in diameter. Figure 3, which depicts a three-dimensional CT reconstruction, delineates the anatomical relationship of the aneurysm with the aortic arch, the left subclavian artery, and the descending thoracic aorta. The aneurysm was localized, with no involvement of the proximal left subclavian artery or its orifice. The distal portion of the aneurysm did not extend to spinal or intercostal branches. The remainder of the aorta, including the abdominal segment, appeared normal. The primary clinical impact of the aneurysm was attributed to a significant mass effect, leading to compression of the trachea and bronchi as well as potential irritation of the left phrenic nerve secondary to mediastinal compression.

The patient was prepared for surgery under standard cardiac anesthesia and positioned in the prone position. A median sternotomy was performed, followed by pericardiectomy. Cannulation of the aorta and right atrium was achieved, and cold crystalloid cardioplegia was administered antegradely via the ascending aorta. Dissection of the aortic arch and descending aorta was undertaken, spanning from the left subclavian artery to the descending thoracic aorta. Critical anatomical structures, including the left phrenic nerve, extensions of the left recurrent laryngeal nerve, and the vagus nerve, were carefully identified and preserved through blunt dissection.
Aneurysmectomy was executed with aortic incision (aortotomy), followed by intraluminal inspection, which confirmed the absence of involvement of spinal arteries or the thoracic segment. Macroscopically, the aneurysm wall appeared thinned and dilated without visible intramural thrombus or dissection flaps. No histological examination of the resected aortic specimen was performed, as it was not deemed necessary for clinical management; the etiology was presumed to be atherosclerotic based on the patient’s risk factors (hypertension, dyslipidemia, smoking), though idiopathic or degenerative causes could not be ruled out. A proximal anastomosis was performed by using a 30 mm Dacron graft with 4-0 Prolene sutures at the border of the left subclavian artery. The distal anastomosis was executed in a continuous fashion with 4-0 Prolene sutures to the descending aorta at the terminus of the aneurysmal segment. Following the completion of aortic grafting, Coronary Artery Bypass Grafting (CABG) was performed, targeting segments S7–S9.
The patient was transferred to the Intensive Care Unit (ICU) for postoperative monitoring. The early postoperative course was complicated by respiratory acidosis, observed on the second postoperative day along with emphysema and pulmonary edema. Hemodynamic instability due to significant blood loss was noted on postoperative day 2, with a total blood loss of 800 mL. Despite the volume loss, resternotomy was not pursued. The patient experienced profound hypovolemia, necessitating vasopressor support with norepinephrine administered as a continuous infusion at a rate of 0.04 μg/kg/min. Following the stabilization of the acid-base balance, the patient was extubated on the second postoperative day.
On the fourth postoperative day, the patient was transferred back to the cardiac surgery department in stable condition. On the tenth postoperative day, the patient was discharged for outpatient follow-up after the completion of the rehabilitation program.
GDTAA, defined as aneurysms exceeding 10 cm in diameter, represents an exceptionally rare and clinically challenging condition, with an estimated prevalence of 10 per 100,000 individuals in the general population [3]. Despite significant advances in diagnostic and therapeutic modalities, GDTAA continues to carry a high risk of morbidity and mortality due to its size and anatomical involvement [1]. The presented case of a 68-year-old male with a 14.08 × 10.04 cm GDTAA exemplifies the critical need for an early detection and timely intervention so that to prevent catastrophic complications such as rupture and dissection [7].
The pathogenesis of GDTAA is associated with degenerative changes in the media, leading to progressive weakening and dilation of the aortic wall under chronic hemodynamic stress [12]. Histological analyses typically indicate that fragmentation of elastic fibers and smooth muscle cell loss contribute to the pathological expansion of the vessel; common features include cystic medial degeneration, atherosclerosis, or inflammation, though, in this particular case, no histology was performed to confirm the assumption [13]. Key risk factors include long-standing hypertension, dyslipidemia, smoking, and genetic predispositions such as Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome [14]. In our case, the history of hypertension and dyslipidaemia associated with aneurysmal growth was exacerbated by chronic B-cell lymphocytic leukaemia, which has been reported to complicate aortic aneurysms through potential infiltration or associated vascular degeneration [15, 16].
GDTAA expansion is influenced by haemodynamic forces that exacerbate elastic fibre degeneration and compromise the vascular smooth muscle integrity [12]. An increased wall stress caused by systemic hypertension and local pressure gradients accelerates pathological remodelling of the aortic wall [17]. Recent studies also suggest that chronic inflammation through macrophage infiltration and cytokine release plays a crucial role in the progression of thoracic aortic aneurysms [18].
GDTAA often presents with non-specific symptoms that are primarily due to mass effect rather than rupture [19]. Common clinical manifestations include dyspnoea, cough, chest pain and dysphagia due to compression of adjacent structures such as the trachea, bronchi and oesophagus [9]. In the case presented, the patient had recurrent syncope, persistent cough and fatigue, which correlated with significant deviation of the trachea and compression of the airway structures on imaging [20].
CT angiography remains the gold standard for the diagnosis and assessment of the extent of GDTAA [21]. It allows high-resolution visualisation of the size and morphology of the aneurysm as well as the anatomical relationships with the adjacent structures [21]. In this case, CT imaging confirmed a massive aneurysm with significant displacement of the trachea and compression of the bronchi, thereby emphasising the crucial role of modern imaging in surgical planning [21].
Surgical intervention is the primary method of treating GDTAA, especially if the aneurysm is larger than 6.5 cm or symptomatic [22]. Open surgical repair, as performed in this case, involves resection of the aneurysmal segment and replacement with a Dacron graft, which has been shown to be durable over time [23]. Thoracic Endovascular Aortic Repair (TEVAR) is a new alternative that is associated with less perioperative morbidity and faster recovery [3]. However, its applicability is limited in cases of an extreme aneurysm size or unfavourable anatomical configurations [24].
In the presented case, open surgical repair was chosen due to the giant size of the aneurysm and its anatomical complexity, which would have posed significant challenges for endovascular stent placement [24]. The simultaneous performance of Coronary Artery Bypass Grafting (CABG) is another example of the need to consider concomitant cardiovascular conditions when repairing an aortic aneurysm [25].
To provide the context, the following table compares the key clinical characteristics of selected published GDTAA case reports with the present case:
|
Reference |
Age/Sex |
Location |
Size (cm) |
Symptoms |
Treatment |
|
Present case |
68/M |
Descending thoracic |
14.08 × 10.04 |
Recurrent syncope, chronic cough, fatigue |
Open repair with Dacron graft + CABG |
|
González-Urquijo et al. (2018) (1) |
Not specified |
Thoracic |
15 |
Asymptomatic |
Endovascular repair |
|
Okura et al. |
Not specified |
Thoracic |
Giant (not quantified) |
Not specified (unruptured) |
Not specified |
|
Oh et al. |
48/M |
Descending thoracic |
12 |
Squeezing chest pain mimicking ACS |
TEVAR |
|
De Mora et al. (2022) (4) |
42/M |
Ascending thoracic and arch |
Giant (not quantified) |
Not specified |
Not specified |
This comparison highlights the variability in presentation and management; our case stands out for its extreme size and the combined open surgical approach in a symptomatic patient with comorbidities.
Postoperative complications following GDTAA repair may include respiratory failure, bleeding, haemodynamic instability and, in rare cases, paraplegia due to spinal cord ischaemia [26, 27]. The patient experienced transient respiratory acidosis, emphysema and pulmonary oedema after the surgery, which were effectively treated and led to a favourable outcome [28]. Despite these challenges, the patient was discharged on the tenth postoperative day, thus emphasising the importance of careful perioperative management [29].
The limited literature on GDTAA highlights the need for centre-specific treatment protocols developed in collaboration with interventional cardiology and adapted to current guidelines. A better understanding of the pathophysiology of GDTAA, including the role of genetic and haematological conditions (such as chronic B-cell lymphocytic leukemia in this case, which may contribute to vascular weakening or complications), is essential for early risk stratification and timely intervention. New imaging techniques such as 3D CT provide valuable insights into anatomical relationships and support more precise diagnosis and treatment planning.
Ethical approval is not required for this study. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
The authors declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
R.Z., Y.O., Se.A.: writing – original draft and review and editing.
R.B., T.L., A.S.: methodology, validation, data curation, supervision, and writing – review and editing.
Si.A.: writing – original draft and data curation.
All data generated during this study are included in this article. Further inquiries can be directed to the corresponding author.
GDTAA – Giant Descending Thoracic Aortic Aneurysm
CABG – Coronary Artery Bypass Grafting
CT – Computed Tomography
TTE – Transthoracic Echocardiography
ICU – Intensive Care Unit
ECG – Electrocardiogram
EF – Ejection Fraction
LVMI – Left Ventricular Mass Index
LVM – Left Ventricular Mass
TEVAR – Thoracic Endovascular Aortic Repair
SVC – Superior Vena Cava
CSF – Cerebrospinal Fluid
3D CT – Three-Dimensional Computed Tomography
LV – Left Ventricle
RV – Right Ventricle
GDTAA – Milžiniška nusileidžiančios krūtinės aortos aneurizma
CABG – Arterijos koronarinės šuntavimas
CT – Kompiuterinė tomografija
TTE – Transtorakalinė echokardiografija
ICU – Intensyviosios terapijos skyrius
ECG – Elektrokardiograma
EF – Išmetimo frakcija
LVMI – Kairiojo skilvelio masės indeksas
LVM – Kairiojo skilvelio masė
TEVAR – Torakalinė endovaskulinė aortos rekonstrukcija
SVC – Viršutinė tuščioji vena
CSF – Cerebrospinalinis skystis
3D CT – Trimatė kompiuterinė tomografija
LV – Kairysis skilvelis
RV – Dešinysis skilvelis