Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2019, vol. 18 (1), pp. 18–22 DOI: https://doi.org/10.15388/LietChirur.2019.18.2
Robotic Cholecystectomy: First Experience in Baltic Countries
Olegas Deduchovas
Department of Surgery, Klaipėda University Hospital, Klaipėda, Lithuania
o.deduchovas@gmail.com
Narimantas Evaldas Samalavičius
Department of Surgery, Klaipėda University Hospital, Klaipėda, Lithuania
Clinic of Internal, Family Medicine and Oncology, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Abstract. In this retrospective study we report the first series of robotic cholecystectomies in Baltic countries. From Nov 2018 to Feb 2019, 13 robotic cholecystectomies were performed in Klaipėda University Hospital using the Senhance (TransEnterix) robotic system. Patients were diagnosed with symptomatic gallstone disease and had no life-threatening co-morbidities. We retrospectively investigated patient demographics and pre-, peri- and postoperative data. Five male and eight female patients were included in this study (n = 13). Mean age was 46 years (range 26–72); mean BMI was 26.7 kg/m² (range 21.1–37.7). Mean docking time was 18 min (range 8–27), and mean operative time was 85 min (range, 70–150). There were no conversions to standard laparoscopy or open surgery. There were no intra-operative complications. There was one post-operative bleeding from the gallbladder bed and subhepatic hematoma, successfully treated by laparoscopy. This study demonstrates the feasibility of robotic surgery in performing minimally invasive cholecystectomies.
Key words: robotic surgery, robotic cholecystectomy, cholecystectomy.
Robotinė cholecistektomija: pirmosios operacijos Baltijos šalyse
Santrauka. Straipsnyje aptariami pirmieji Baltijos šalyse robotinės cholecistektomijos rezultatai. Operacijos atliktos Klaipėdos universitetinėje ligoninėje nuo 2018 m. lapkričio mėn. iki 2019 m. vasario mėn., naudojant Senhance (TransEnterix) robotinę sistemą. Tiriamieji sirgo simptomine tulžies pūslės akmenlige, sunkių gretutinių susirgimų neturėjo.
Atliekant tyrimą, retrospektyviai įvertinti pacientų demografiniai duomenys, operaciniai ir pooperaciniai rezultatai. Tyrime dalyvavo 13 pacientų: penki vyrai ir aštuonios moterys. Pacientų amžiaus vidurkis – 46 metai (26–72 m.), KMI vidurkis – 26,7 kg/m² (21,1–37,7 kg/m²). Paruošti operacijai robotinę sistemą vidutiniškai užtruko 18 min. (8–27 min.). Robotinė cholecistektomija (kartu su pasiruošimu) vidutiniškai truko 85 min. (70–150 min.). Konversijų į laparoskopinę ar atvirą operaciją nebuvo. Operacinių komplikacijų nefiksuota. Vienam pacientui po operacijos pastebėta kraujavimo iš tulžies pūslės guolio požymių ir subhepatinė hematoma, tačiau pacientas sėkmingai pagydytas relaparoskopijos metu.
Straipsnyje aptariami tyrimo duomenys atskleidžia robotinės chirurgijos galimybes tulžies pūslės akmenligei gydyti.
Reikšminiai žodžiai: robotinė chirurgija, robotinė cholecistektomija, cholecistektomija.
Received: 2019/04/05. Accpeted: 2019/04/22
Copyright © 2019 Olegas Deduchovas, Narimantas Evaldas Samalavičius. Published by Vilnius University Press
This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
In 1882, Carl Langebuch (1846–1901) of Germany performed the first cholecystectomy [1]. In 1985 (103 years later), Erich Mühe of Germany performed the first laparoscopic cholecystectomy, following by Phillipe Mouret (1987). Because of its advantages (smaller incisions, quicker recovery time, improved cosmetic results and shorter hospital stay), became accepted within a few years as the new standard therapy for gallstone disease [1]. The first robotic-assisted cholecystectomy was performed in 1997 and since then many reports on robotic cholecystectomy have been published [2]. All authors agreed on the safety and feasibility of the robotic procedure. However, most of them concluded that this procedure is not acceptable as a standard operation because of the lack of benefits for patients due to the high cost and prolonged operating time. In this regard, the benefits of the robotic procedure in gallbladder diseases have not yet been established [3–4]. However, until recently, robotic-assisted surgery has exclusively been connected to the name DaVinci®. In 2016, a second robotic system, the Senhance®, became available [5]. Because of its safety and possibility to resterilize the instruments of Senhance robotic system, it became feasible to perform smaller routine operations like robotic cholecystectomy [6].
Materials and methods
From November 2018 to February 2019, a total of 13 robotic cholecystectomies were performed at Klaipėda University Hospital. We prospectively collected the docking time and console time in all robotic procedures. The initial indications of surgery included symptomatic gallstones. Exclusion criteria were the presence of acute cholecystitis and previous history of extensive upper abdominal surgery. Informed consent was obtained for the robotic cholecystectomy. We retrospectively reviewed the medical records of all patients and analyzed data, including demographic information, clinical presentation, results of laboratory studies, operative records, postoperative complications, and postoperative hospital stay.
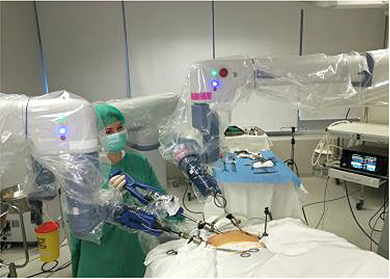
Figure 1. Docking of robotic arms

Figure 2. Robotic dissection in process
In this study, the operating time was defined as the time from skin incision to wound closure. The docking time spanned the setup of the robot onto the surgical field. The console time was defined as the time from the start of dissection until the moment the gallbladder was completely freed from the liver.
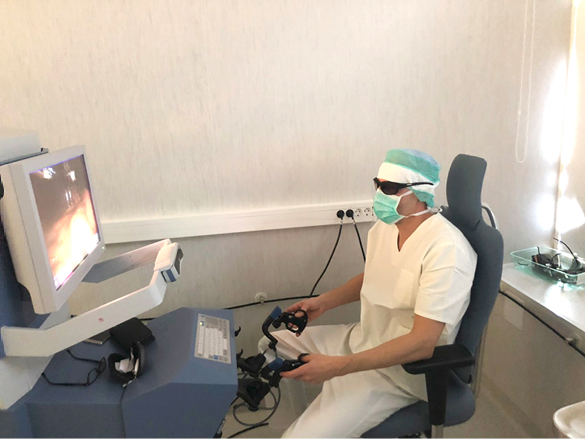
Figure 3. Surgeon works at console
The robotic-assisted operations were performed with the Senhance system (TransEnterix, Inc., Morrisville, NC, USA). We participated in a 4-day intensive training program with this system at the European training center of TransEnterix Inc. in Milan. Surgeons and nurses of our team were able to use the robot over several hours. The training was concluded with procedural performances in an animal model, a test, and a certificate being awarded. The operating team consisted of one operating surgeon, one assisting surgeon and the certified nurse. The assisting surgeon replaced instruments and paced clips during cholecystectomy. Robotic cholecystectomy was performed using a four port technique (Figure 1). First, a 10-mm trocar was inserted through an incision over the umbilicus using a close method. CO2 gas was introduced through this trocar to obtain an intraperitoneal pressure of 12 mm Hg. All other ports were placed under direct visualization. The 10 and 5 mm ports were placed about 8–10 cm on the right and on the left from the umbilicus, respectively. An additional fourth trocar (5 mm) was placed in the right anterior axillary line in the upper quadrant and used for retraction and suction by assistant.
Table 1. Demographic data
|
Robotic cholecystectomy (n = 13) |
|
Age (yr) 46±11.0 |
|
Gender (male/female) 5/8 |
|
Previous ERCP and bile stones extraction – |
|
BMI 26.7 kg/m2 (range 21.1–37.7) |
|
White blood cell count 7.4±2.8 |
|
Diabetes mellitus 1 |
|
Hypertension 2 |
The patient was then placed in reverse Trendelenburg position with the right side up. The Senhance TransEnterix surgical robot was then brought into position and docked. When performing cholecystectomy with the Senhance, TransEnterix robotic system, 3 independently usable robotic arms are used (Figure 1). To prepare, we regularly use a monopolar hook (right hand/right robotic arm) and a bipolar grasper (left hand/left robotic arm) (Figure 2). The third arm is used as a camera holder. An integrated 3D camera with 16-fold magnification offers a very high-quality visible field and precise assessment of thinnest tissue structures. With ‘Eye-Sensing Control’, the camera can be maneuvered precisely by the eye movements of the surgeon after the initial calibration from the console (Figure 3). The dissection was performed according to the standard laparoscopic technique. After clear identification of the cystic duct and cystic artery, the cystic duct was ligated manually with clips. The cystic artery was coagulated or clipped just around the gallbladder. The gallbladder was dissected from the bed. Once fully dissected, the gallbladder was removed through the umbilical 18 mm port. The robot was then withdrawn, and the 18 mm port site was closed with absorbable sutures. Finally, the skin incisions at the port sites were sutured.
Table 2. Operation details
|
Operation time (min) 84.6±18.1 (docking time + console time + finishing and suturing time) |
|
Docking time (min) 18±8 min (8–27 min) |
|
Console time (min) 34.5±12.0 (25–80 min) |
Table 3. Surgical outcomes of robotic cholecystectomy
|
Postoperative bleeding |
1 |
|
Bile duct injury |
0 |
|
Laparoscopic conversion |
0 |
|
Open conversion |
0 |
|
Total hospital stay (d) |
1.8±1.2 |
|
Postoperative hospital stay (d) |
1.2±1.1 |
Patients were discharged on the first or second day after surgery if sufficiently recovered and if pain and nausea had receded. All patients were seen for examination and reassessment at the outpatient clinics 1 week after surgery. Laboratory tests were performed only if indicated.
Five patients were male and 8 female; the age ranged from 26 to 72 years of age (mean 46±11.0 years). Table 1 shows the clinical characteristics of patients who underwent robotic cholecystectomy. The associated diseases were hypertension (n = 2), diabetes mellitus (n = 1). The previous operations were appendectomy (n = 1), and hysterectomy (n = 1). Endoscopic retrograde cholangiopancreatogram (ERCP) and biliary stones extraction were performed in 8 of 13 patients (62%) in the period from one week to two months before surgery. After robotic cholecystectomy, all patients were diagnosed with gallbladder stones.
Results
All robotic procedures were successfully completed. The mean operation time was 84.6±18.1 min. The docking time and console time were 18±8 min (8–27 min) and 34.5±12.0 min (25–80 min), respectively (Table 2). The conversion rate to laparoscopic or open procedures was zero. The complication rates was 7.6% (n = 1, bleeding and postoperative hematoma) (Table 3). The patient who had complication was a 58-year-old man who was discharged on the first post operative day, but was readmitted and underwent re-laparoscopy on postoperative day 4; previous incisions on the low abdominal area were employed during the surgery. We identified the focus of bleeding on the gallbladder bed and coagulated the bleeder. The patient was finally discharged from the hospital without any symptoms. There was no bleeding associated with the cystic artery. There was no bile duct injury and mortality. The mean postoperative hospital stay was 1.2±1.1 d.
Discussion
The worldwide number and interest of robotic-assisted surgeries is growing in the recent past years. In abdominal surgery, robotic-assisted surgery has so far only been used in selected complex cases, mostly because of the high costs and the comparably long process times. In a prospective case-matched study Breinstein et al. [7] concluded that, while RC was safe and valuable, they were unable to justify its use because of the high cost of the robotic system. The authors found no added benefits to the patients versus laparoscopic cholecystectomy (LC). Heemskerk et al., in 2005 reported similar findings in a series of 24 patients [7]. A significantly longer operative time than LC with no advantage from robotic assistance were reported previously [4, 8]. However, Zhou et al., in their series of 40 patients, found that robotic assistance provided better control of the operative field and had the advantage of increased precision and stability when compared with LC [9]. The introduction of a Senhance TransEnterix robotic system has created new feasibility for robotic cholecystectomy, because of the safety and reduced per-case costs [5–6]. Especially with a high case load, this reduction is significant. All instruments of this robotic platform are resterilizable and standard trocars are used. Therefore, especially during the learning curve, it became feasible to perform smaller routine operations like RC.
In our study we have experienced one complication, postoperative bleeding (7.6%). The reported incidence of bleeding in laparoscopic cholecystectomy can be up to 2% (reported range, 0.03% to 10%). Despite of the high rate of postoperative bleeding in our study, we strongly believe that with the expanding of RC patient number, the rate of the latter complication will become acceptable.
We also have experienced the benefits for surgeons in the area of ergonomic during operation including a comfortable and relaxed seating position (Figure 3). In traditional laparoscopy, the operating surgeon is dependent on the experience of the assistant and his/her camera steering. In this context, a special advantage of robotic-assisted surgery may be comfortable ergonomics, a 3-dimensional (3D) view of the operating field, up to 16-fold magnification, and stable camera positioning which automatically compensates for unwanted camera movements. Further studies need to be performed to verify advantages and disadvantages of the robotic cholecystectomy compared to laparoscopic surgery.
References
1. Reynolds W Jr. The First Laparoscopic Cholecystectomy. JSLS 2001; 5(1): 89–94.
2. Solano C, Gualtierotti M, Cahill R, Marescaux J. Robot-assisted Cholecystectomy. Biliary Lithiasis 2008; 209–216. https://doi.org/10.1007/978-88-470-0763-5_17
3. Strosberg DS, Nguyen MC, Muscarella P, Narula VK. A retrospective comparison of robotic cholecystectomy versus laparoscopic cholecystectomy: operative outcomes and cost analysis. Surgical Endoscopy 2016; 31: 304–307. https://doi.org/10.1007/s00464-016-5134-0
4. Nio D, Bemelman WA, Busch OR, Vrouenraets BC, Gouma DJ. Robot-assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy: a comparative study. Surg Endosc. 2004; 18: 379–382. https://doi.org/10.1007/s00464-003-9133-6
5. Stephan D, Sälzer H, Willeke F. First Experiences with the New Senhance® Telerobotic System in Visceral Surgery. Visc Med. 2018 Feb; 34(1): 31–36. https://doi.org/10.1159/000486111
6. Melling N, Barr J, Schmitz R, Polonski A, Miro J, Ghadban T, Wodack K, Izbicki J, Zani S, Perez D. Robotic cholecystectomy: first experience with the new Senhance robotic system. J Robot Surg. 2018; Sep 27: 701–704. https://doi.org/10.1007/s11701-018-0877-3
7. Breitenstein S, Nocito A, Puhan M, Held U, Weber M, Clavien PA. Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study. Ann. Surg. 2008; 247: 987–9 93. https://doi.org/10.1097/sla.0b013e318172501f
8. Kornprat P, Werkgartner G, Cerwenka H, Bacher H, El-Shabrawi A, Rehak P. Prospective study comparing standard and robotically assisted laparoscopic cholecystectomy. Langenbecks Arch. Surg. 2006; 391: 216–221. https://doi.org/10.1007/s00423-006-0046-4
9. Zhou HX, Guo YH, Yu XF, Bao SY, Liu JL, Zhang Y. Zeus robot-assisted laparoscopic cholecystectomy in comparison with conventional laparoscopic cholecystectomy. Hepatobiliary Pancreat. Dis. Int. 2006; 5: 115–118.
10. Kaushik R. Bleeding complications in laparoscopic cholecystectomy: Incidence, mechanisms, prevention and management. Journal of Minimal Access Surgery 2010; 6: 59–65. https://doi.org/10.4103/0972-9941.68579