Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2019, vol. 18(4), pp. 239–245 DOI: https://doi.org/10.15388/LietChirur.2019.18.16
Does the Chest Tube Removal Time Influence the Outcomes of Primary Spontaneus Pneumothorax First Episode Management?
Matas Mongirdas
Vilniaus universiteto Medicinos fakultetas, Lietuva
Faculty of Medicine, Vilnius University, Lithuania
Audrius Untanas
Vilniaus universiteto Medicinos fakultetas, Lietuva
Faculty of Medicine, Vilnius University, Lithuania
Žymantas Jagelavičius
Krūtinės chirurgijos centras, Krūtinės ligų, imunologijos ir alergologijos klinika, Klinikinės medicinos institutas, Vilniaus universiteto Medicinos fakultetas, Lietuva
Centre of Thoracic Surgery, Clinic of Chest Diseases, Immunology and Allergology, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Lithuania
El. paštas zymant@yahoo.com
Ričardas Janilionis
Krūtinės chirurgijos centras, Krūtinės ligų, imunologijos ir alergologijos klinika, Klinikinės medicinos institutas, Vilniaus universiteto Medicinos fakultetas, Lietuva
Centre of Thoracic Surgery, Clinic of Chest Diseases, Immunology and Allergology, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Lithuania
Abstract. Background / objectives. The main treatment option for the first episode of primary spontaneous pneumothorax is chest tube drainage, however, whether delayed chest tube removal might influence the recurrence is unclear.
Methods. A prospective study, which included 50 patients, with an initial episode of primary spontaneous pneumothorax was performed. Patients were randomized into two groups according to the chest tube removal time: 1-day and 5-days after the air-leak has stopped. All patients were followed-up for at least six months. Both groups were compared according to the recurrence rate and possible complications.
Results. There were 39 (78%) men and the median age was 27 (23–35) years. Successful management with a chest tube was achieved in 43 (86%) patients, others were operated on because of the continuous air-leak or relapse of the pneumothorax after the chest tube was removed. Significant difference was not found comparing groups by age, gender, side, tobacco smoking, alpha-1-antitrypsin level, rate of prolonged air-leak, necessity of surgery, and the mean follow-up time. There was a significant difference between groups in hospitalization time: 1-day group – 6 (4–12), 5-days group – 8 (7–10) days, p = 0.017. Five (20%) patients from 1-day group and 3 (12%) from 5-days group had a recurrence, however the difference was not significant (p = 0.702). There were no significant differences comparing groups by the recurrence time or complications.
Conclusions. The recurrence rate of primary spontaneous pneumothorax was higher if the chest tube was removed earlier, however not significantly. More data and longer follow-up are necessary to confirm these findings.
Key words: primary spontaneous pneumothorax, chest tube, chest tube removal, recurrence rate, complications.
Ar dreno pašalinimo laikas turi įtakos pirminio spontaninio pneumotorakso pirmojo epizodo gydymo išeitims?
Santrauka. Įvadas / tikslas. Pirminis spontaninis pneumotoraksas yra didelė jaunų sveikų žmonių problema. Pleuros ertmės drenavimas – pagrindinis pirminio spontaninio pneumotorakso pirmojo epizodo gydymo būdas. Vis dėlto nėra žinoma, ar dreno buvimo pleuros ertmėje trukmė gali turėti įtakos recidyvų dažniui.
Metodai. Atliktas perspektyvusis tyrimas, į kurį įtraukta 50 pacientų. Tiriamiesiems diagnozuotas pirminio spontaninio pneumotorakso pirmasis epizodas. Visi pacientai gydyti drenuojant pleuros ertmę. Pacientai suskirstyti į dvi grupes, atsižvelgiant į dreno pašalinimo laiką: praėjus vienai dienai ar praėjus penkioms dienoms, kai per dreną nustojo skirtis oras. Visi pacientai atokiu laikotarpiu (mažiausiai šešis mėnesius) stebėti dėl galimo recidyvo. Atlikta abiejų grupių recidyvų dažnio ir galimų komplikacijų lyginamoji analizė.
Rezultatai. Vidutinis pacientų amžius – 27 (23–35) metai. 78 proc. tiriamųjų – vyrai. Drenavus pleuros ertmę, sėkmingai išgydyti 43 ligoniai (86 %). Kiti tiriamieji dėl besiskiriančio per dreną oro ar dėl rentgenogramoje matomo pakartotinio pneumotorakso, pašalinus dreną, buvo operuoti. Abiejų tiriamųjų grupių duomenys pagal amžių, lytį, pneumotorakso pasireiškimo krūtinės ląstoje pusę, rūkymą, alfa-1 antitripsino koncentraciją kraujyje, ilgesnį oro skyrimąsi per dreną ar operacijos poreikį statistiškai reikšmingai nesiskyrė. Statistiškai reikšmingai skyrėsi abiejų grupių hospitalizacijos trukmė: tiriamieji, kuriems, nustojus per dreną skirtis orui, drenas pašalintas praėjus vienai dienai, ligoninėje gulėjo vidutiniškai 6 (4–12) dienas, o tiriamieji, kuriems drenas pašalintas praėjus penkioms dienoms, – 8 (7–10) dienas (p = 0,017). Penkiems (20 %) pirmosios grupės ir trims (12 %) antrosios grupės pacientams pneumotoraksas recidyvavo (skirtumas statistiškai nereikšmingas (p = 0,702)). Statistiškai nereikšmingas skirtumas nustatytas ir tarp abiejų grupių recidyvo laiko bei komplikacijų.
Išvados. Pirminio spontaninio pneumotorakso recidyvų dažnis kiek didesnis, kai drenas ištraukiamas anksčiau, tačiau lyginamųjų grupių skirtumas statistiškai nereikšmingas. Siekiant patvirtinti gautus rezultatus, reikia atlikti didesnės apimties tyrimą, būtina ilgiau stebėti tiriamuosius.
Reikšminiai žodžiai: pirminis spontaninis pneumotoraksas, pleuros ertmės drenavimas, pleuros dreno pašalinimas, recidyvų dažnis, komplikacijos.
Received: 2019/09/20. Accepted: 2019/10/31.
Copyright © 2019 Matas Mongirdas, Audrius Untanas, Žymantas Jagelavičius, Ričardas Janilionis. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Pneumothorax is defined as the presence of air in the pleural cavity leading to loss of the negative sub-atmospheric intrapleural pressure and lung collapse. Spontaneous pneumothorax occurs without any reason, usually at rest and can be classified as primary, when there is no apparent lung disease or secondary, which is related to known underlying lung disease [1, 2].
Primary spontaneous pneumothorax (PSP) is a significant health problem and has an age-adjusted incidence of 7.4 to 18 cases per 100 000 population per year in males, and 1.2 to 6 cases per 100 000 population per year in females [3]. It usually occurs in tall, thin males and it is associated with an asthenic or leptosomic physical constitution. Other risk factors include cigarette smoking, congenital disorders such as Marfan’s syndrome and some environmental factors such as atmospheric pressure changes [4, 5].
Usually the first episode of PSP is successfully managed with a chest tube drainage. However, in up to 42% of patients it may recur [6] and then it should be managed surgically to prevent further recurrence. It is unclear how to prevent the recurrence of PSP after the first episode managed with a chest tube drainage. Potentially, a chest tube left for a longer time in the pleural cavity may reduce the incidence of recurrence. The aim of this study was to evaluate whether chest tube time influence the recurrence rate of PSP and whether it is associated with the higher complication rate.
Methods
Fifty patients with the first episode of primary spontaneous pneumothorax were included in the prospective study during the period from March 2016 to October 2018. All diagnoses were made by the clinical picture and confirmed with an anteroposterior chest X-ray. All patients were managed primarily with a chest tube drainage.
Data including demographic (age, sex), risk factors (tobacco smoking, alpha-1 antitrypsin AAT) level), the side of pneumothorax, hospitalization time, possible complications, prolonged air-leak and necessity of surgery was recorded. Prolonged air-leak was defined as an air-leak lasting longer than 5 days. Those patients where the chest tube drainage was unsuccessful (either because of continuous air-leak or pneumothorax on the control chest X-ray after chest tube removal), went for surgery during the same hospitalization period.
Patients were randomized into two groups, according to the chest tube removal time: 1-day group – chest tube was removed the next day after the air-leak has stopped and 5-days group – chest tube was removed on the 5th day after the air-leak has stopped. A control chest X-ray was performed in every patient at least twice: the next day after the chest tube was inserted and the next day after it was removed. All patients were followed-up for at least six months with a mean follow-up time 23±8 months. Recurrences of PSP and possible early or late complications were recorded. To evaluate homogeneity of groups, they were compared according to demographic data, pneumothorax side, risk factors, and follow-up time. Later groups were compared according to the hospitalization time, possible complications and recurrence rate.
A recurrent pneumothorax was defined as the presence of a new spontaneous pneumothorax episode confirmed by the chest X-ray after the discharge with a complete resolution of the previous pneumothorax episode.
Statistical analysis was performed using SPSS 25.0 for Windows (SPSS Inc. Chicago, IL, USA). Kolmogorov-Smirnov test was used to check if continuous data is distributed normally. The Chi-square and Fisher exact tests were used to compare categorical variables, t-test and Mann-Whitney test were used to compare continuous variables. Continuous data that was normally distributed was reported as mean ± standard deviation (SD), non-normally distributed continuous data was reported as median and quartile range (QR). A statistical significance level was set at 0.05.
Results
Fifty patients with the first episode of PSP were included in the study. The male gender was the prevailing – 39 patients (78%), male to female ratio was 3.5:1. Age varied from 18 to 48 years and the median was 27 (23–35) years (Fig. 1).
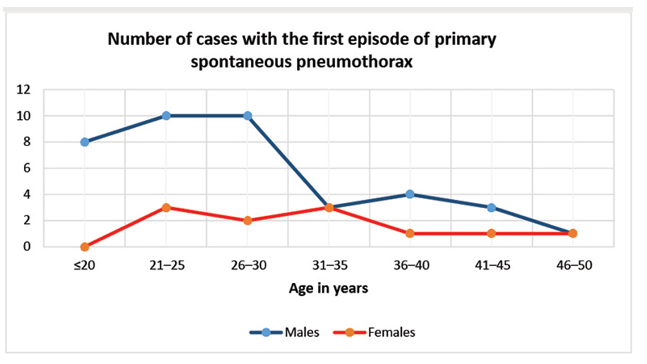
Figure 1. Comparison of age distribution in males and females
One case was bilateral at presentation (the patient was managed with a bilateral chest tube drainage) and 49 were unilateral (26 left sided and 23 right sided).
Thirty-three (66%) patients had a history of tobacco smoking. Median lifetime tobacco exposure was 6 (2–10) pack years. One patient had a lower AAT level (0.76 g/L). The normal value was considered as 0.9–1.9 g/L. The mean AAT concentration was 1.48±0.32 g/L.
Comparing two groups by age, gender, lesion side, tobacco smoking, AAT level, and mean follow-up time no statistically significant differences were found (Table 1). Results showed that both groups were homogenous.
Table 1. Comparison of the groups according the basic data
|
Factor |
1-day (n = 25) |
5-days (n = 25) |
p-value |
|
Age |
28 (23–39) |
27 (25–31) |
0.698 |
|
Gender: male |
18 (72%) |
21 (84%) |
0.306 |
|
Side: right |
9 (36%) |
15 (60%) |
0.089 |
|
Tobacco smoking |
15 (60%) |
18 (72%) |
0.370 |
|
Tobacco smoking (packyears) |
6 (2.5–13) |
5.5 (2–10) |
0.758 |
|
AAT level (g/l) |
1.48±0.32 |
1.47±0.32 |
0.929 |
|
Mean follow-up time (days) |
685±242 |
698±267 |
0.867 |
Successful management with a chest tube was achieved in 43 (86%) patients, seven patients were operated on after the unsuccessful management with the chest tube. The most common indication for operation was continuous air-leak. The rate of prolonged air-leak and necessity of surgery did not significantly differ between the groups (Table 2). Three patients (one in 1-day and two in 5-days group) had infectious complications, such as chest tube wound infection, pneumonia, and influenza, however, there was no significant difference (p = 1.0) between the groups.
Table 2. Comparison of the groups according treatment outcomes
|
Factor |
1-day group (n = 25) |
5-days group (n = 25) |
p-value |
|
Air-leak >5 days |
8 (32%) |
6 (24%) |
0.529 |
|
Necessity of surgery |
4 (16%) |
3 (12%) |
1.000 |
|
Length of stay (days) |
6 (4–12) |
8 (7–10) |
0.017 |
|
Infectious complications |
1 (4%) |
2 (8%) |
1.0 |
|
Same side recurrence rate |
5 (20%) |
3 (12%) |
0.702 |
|
Same side recurrence time (days) |
113 (31–515) |
33 (30–422) |
0.881 |
The median length of stay significantly differed between groups: 6 (4–12) days in 1-day group and 8 (7–10) days in 5-days group, p = 0.017.
During the follow-up, 10 episodes of recurrence in 10 (20%) patients were observed. In eight (16%) of them the recurrence occurred on the same side as the primary episode. The median recurrence time on the same side was 73 (27–640) days. The same side recurrence rate was higher in 1-day group (5 out of 25 (20%) vs 3 out of 25 (12%)), however, the difference was not statistically significant as well as comparing recurrence time (Table 2).
Neither late complications nor deaths were observed.
Discussion
Although “pneumothorax” was described by Laennec in the beginning of 19th century [7], spontaneous pneumothorax remains a common condition that significantly affects health care nowadays. Plenty of factors are involved in the pathogenesis of PSP. Most authors state that subpleural bullae rupture and air leakage into the pleural space is the main pathophysiological reason of PSP [8]. Amjadi et al. in their study noticed that only a part of all patients with PSP could be found with blebs or bullae in imaging or even at the time of surgery [9]. The development of blebs may be related to variety of factors such as smoking (due to local emphysema development), hereditary predisposition, low body mass index (because of greater distending pressure at the apex of the thorax) and caloric restriction, AAT deficiency and some environmental factors such as atmospheric pressure changes [5, 9–11]. Smoking cigarettes increases the risk of development PSP approximately 9-fold in woman and 22-fold in men [12]. Our study showed 66% of patients had current history of tobacco use. It is also frequently assumed that individuals with severe AAT deficiency have at least a 20-fold increase in the risk of developing lung disease [13]. However, our study revealed that only one patient out of 50 had lower than normal AAT level (0.76 g/L).
Primary spontaneous pneumothorax is the disease of young males. Male to female ratio varies from 2.9 to 10.0 [14–17]. In our study it was 3.5. There is a tendency, that males with PSP are younger than females [14]. The same tendency was observed in our study (Fig.1).
Usually PSP happens one side without a difference between right or left. The literature describes rare cases of bilateral PSP which are usually managed surgically [2, 15, 18]. We had one patient who was successfully managed with bilateral chest tube drainage without a recurrence for already 23 months.
The main goals in the management of PSP are to re-expand the lung and prevent recurrence, with minimal morbidity [19]. There are different therapeutic options described in literature for managing PSP: observation, air aspiration, placement of the chest tube, pleurodesis, thoracoscopy, and thoracotomy [20–22]. Chest tube drainage still remains the main option to manage the first episode of PSP. With a proper chest tube drainage, the lung expands rapidly and the air-leak usually stops in 48 hours. However, prolonged air-leak after chest tube drainage is possible and sometimes leads to surgery [23–25]. In a study by Sayar et al. prolonged air-leak was observed in 15% of patients and all of them were operated on [23]. Chee et al. reported prolonged air-leak in 25% of PSP patients [25]. Light recommended maintenance of suction drainage for a period of 5 to 7 days if there is an air-leak [20]. In our study 28% of patients had a prolonged air-leak (>5 days), all of them was managed with permanent suction via chest tube and only half of them (14%) were operated on, mostly because of a continuous air-leak.
There is a debatable question on when the chest tube should be removed. Everybody agrees that the lung should be expanded (on the X-ray) and there should be no air-leak before removing a chest tube. However, it is unclear on whether we should remove it immediately after air-leak has stopped or should we wait for couple of hours or even days to better prevent early and late recurrences. According to studies by Davis et al. and Seremetis et al., immediate relapse after removal of the chest tube is not uncommon [26, 27]. Sharma et al. reported early recurrence rate of 25% when the chest tube was removed within 6 hours after lung expansion and 0% when the chest tube was removed 48 hours after lung expansion [28]. So and Yu stated, that chest tube should be removed after the air-leak has stopped preferably for 48 hours [29]. Nowadays, usually it is recommended to wait with the removal at least for 24 hours after re-expansion of a lung and cessation of an air-leak.
How to prevent recurrence of PSP without surgery is unclear. There is a hypothesis that chest tube may irritate the pleural surface and may work like mechanical pleurodesis [14, 30, 31]. This probably could explain findings in some studies which showed that the recurrence rate after conservative management (without any intervention or only needle aspiration) is higher comparing to chest tube drainage [14, 21, 22, 27].
In our study the ipsilateral recurrence rate was higher when the chest tube was removed earlier (20% in the group where the chest tube removed 1 day after air-leak has stopped vs 12% in the group where chest tube removed 5 days after the air-leak has stopped), although the difference was not significant. To the best of our knowledge there is no data on whether delayed chest tube removal has an influence on late recurrence of PSP. Our study is quite moderate (50 patients) and the mean follow-up time is 23 months (at least 6 months).
The recent large systematic review and meta-analysis on PSP recurrence after nonsurgical management (conservative management, needle aspiration, chest tube drainage) showed that the estimated recurrence rate was 32% and varied from 8% up to 74% [30]. The risk of PSP recurrence after chest tube drainage is up to 42% [6]. In our study the total recurrence rate was 20%. Ipsilateral recurrence was observed in 78% to 79% of all recurrences [14, 16]. In our study it composes 80% of all recurrences.
Most of the recurrences occur within the first year after the initial episode of PSP [14, 16, 21]. Olesen et al. [14] revealed that 72% of all recurrences occur within the first year, Hofmann et al. [16] showed, that two-third of recurrences occur within the first 6 months. The same tendency was observed in our study (70% of all recurrences occurred within the first year). However, it can be thought that the recurrence rate in our study might be increased after longer follow-up.
Conclusion
The recurrence rate of primary spontaneous pneumothorax was higher when the chest tube was removed earlier comparing to the delayed removal, however, the difference was not significant. Delayed chest tube removal was not associated with a higher incidence of complications, but prolonged hospital stays. More data and longer follow-up are needed to confirm these findings.
References
1. Hallifax RJ, Laskawiec-Szkonter M, Rahman NM; RAMPP Trial collaborators. Predicting outcomes in primary spontaneous pneumothorax using air leak measurements. Thorax 2019; 74: 410–412.
2. Chen YJ, Luh SP, Hsu KY, Chen CR, Tsao TC, Chen JY. Video-assisted thoracoscopic surgery (VATS) for bilateral primary spontaneous pneumothorax. J. Zhejiang Univ Sci B 2008; 9: 335–340.
3. Noppen M, de Keukeleire T. Pneumothorax. Respiration 2008; 76: 121–127.
4. Alifano M, Forti Parri SN, Bonfanti B, Arab WA, Passini A, Boaron M, Roche N. Atmospheric pressure influences the risk of pneumothorax: beware of the storm! Chest 2007; 131: 1877–1882.
5. Roman M, Weinstein A, Macaluso S. Primary spontaneous pneumothorax. Medsurg Nurs 2003; 12: 161–169.
6. Chambers A, Scarci M. In patients with first-episode primary spontaneous pneumothorax is video-assisted thoracoscopic surgery superior to tube thoracostomy alone in terms of time to resolution of pneumothorax and incidence of recurrence? Interact Cardiovasc Thorac Surg 2009; 9: 1003–1008.
7. Roguin A. Rene Theophile Hyacinthe Laënnec (1781–1826): The Man Behind the Stethoscope. Clin Med Res 2006; 4: 230–235.
8. Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000; 342: 868–874.
9. Amjadi K, Alvarez GG, Vanderhelst E, Velkeniers B, Lam M, Noppen M. The prevalence of blebs or bullae among young healthy adults: a thoracoscopic investigation. Chest 2007; 132: 1140–1145.
10. Morrison PJ, Lowry RC, Nevin NC. Familial primary spontaneous pneumothorax consistent with true autosomal dominant inheritance. Thorax 1998; 53: 151–152.
11. Huang TW, Lee SC, Cheng YL, Tzao C, Hsu HH, Chang H, Chen JC. Contralateral recurrence of primary spontaneous pneumothorax. Chest 2007; 132: 1146–1150.
12. Hobbs B, Foreman M, Bowler R, Jacobson F, Make B, Castaldi P, San José Estépar R, Silverman E, Hersh C. Pneumothorax Risk Factors in Smokers with and without Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2014; 11: 1387–1394.
13. Ranes J, Stoller JK. A review of α-antitrypsin deficiency. Semin Respir Crit Care Med 2005; 26: 154–166.
14. Olesen WH, Lindahl-Jacobsen R, Katballe N, Sindby JE, Titlestad IL, Andersen PE, Licht PB. Recurrent Primary Spontaneous Pneumothorax is Common Following Chest Tube and Conservative Treatment. World J Surg 2016; 40: 2163–2170.
15. Noh D, Lee S, Haam SJ, Paik HC, Lee DY. Recurrence of primary spontaneous pneumothorax in young adults and children. Interact Cardiovasc Thorac Surg 2015; 21: 195–199.
16. Hofmann HS, Suttner T, Neu R, Potzger T, Szöke T, Grosser C, Ried M. Burden between Undersupply and Overtreatment in the Care of Primary Spontaneous Pneumothorax. Thorac Cardiovasc Surg 2018; 66: 575–582.
17. Hallifax RJ, Rahman NM. Epidemiology of pneumothorax – finally something solid out of thin air. Thorax 2015; 70: 921–922.
18. Kewcharoen J, Morris P, Kanitsoraphan C, La H, Sriratanaviriyakul N. Simultaneous Bilateral Primary Spontaneous Pneumothorax: A Case Report and a Review of the Literature. Case Rep Pulmonol 2019. https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A6407.
19. Tschopp JM, Rami-Porta R, Noppen N, Astoul P. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006; 28: 637–650.
20. Light RW. Management of Spontaneous Pneumothorax. Am Rev Respir Dis 1993; 148: 245–248.
21. Elsayed HH. Is chest tube drainage losing ground in management of patients with spontaneous pneumothorax? J Thorac Dis 2017; 9: 3518–3522.
22. Kim MJ, Park I, Park JM, Kim KH, Park J, Shin DW. Systematic review and meta-analysis of initial management of pneumothorax in adults: Intercostal tube drainage versus other invasive methods. PLoS One 2017; 12: e0178802. https://doi.org/10.1371/journal.pone.0178802.
23. Sayar A, Kök A, Citak N, Metin M, Büyükkale S, Gürses A. Size of pneumothorax can be a new indication for surgical treatment in primary spontaneous pneumothorax: a prospective study. Ann Thorac Cardiovasc Surg 2014; 20: 192–197.
24. Brown SG, Ball EL, Macdonald SP, Wright C, McD Taylor D. Spontaneous pneumothorax; a multicentre retrospective analysis of emergency treatment, complications and outcomes. Intern Med J 2014; 44: 450–457.
25. Chee CB, Abisheganaden J, Yeo JK, Lee P, Huan PY, Poh SC, Wang YT. Persistent air-leak in spontaneous pneumothorax–clinical course and outcome. Respir Med 1998; 92: 757–761.
26. Davis JW, Mackersie RC, Hoyt DB, Garcia J. Randomized study of algorithms for discontinuing tube thoracostomy drainage. J Am Coll Surg 1994; 179: 553–557.
27. Seremetis MG. The management of spontaneous pneumothorax. Chest 1970; 57: 65–68.
28. Sharma TN, Agnihotri SP, Jain NK, Madan A, Deopura G. Intercostal tube thoracostomy in pneumothorax: factors influencing re-expansion of lung. Indian J Chest Dis Allied Sci 1988; 30: 32–35.
29. So SY, Yu DY. Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax 1982; 37: 46–48.
30. Walker SP, Bibby AC, Halford P, Stadon L, White P, Maskell NA. Recurrence rates in primary spontaneous pneumothorax: a systematic review and meta-analysis. Eur Respir J 2018; 52. PII: 1800864. https://doi.org/10.1183/13993003.00864-2018.
31. Andrivet P, Djedaini K, Teboul JL, Brochard L, Dreyfuss D. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest 1995; 108: 335–339.