Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2021, vol. 20(3–4), pp. 171–176 DOI: https://doi.org/10.15388/LietChirur.2021.20.50
Computed Tomography-Guided Percutaneous Lung Biopsy Complicated by Symptomatic Systemic Air Embolism: Case Report and Review of the Literature
Giedrius Ledas
Faculty of Medicine, Vilnius University, Vilnius, Lithuania
E-mail: giedriusledas@gmail.com
Donatas Jocius
Department of Radiology, Nuclear Medicine and Physics of Medicine, Vilnius University, Vilnius, Lithuania
E-mail: donatas.jocius@gmail.com
Juozas Jarašūnas
Department of Radiology, Nuclear Medicine and Physics of Medicine, Vilnius University, Vilnius, Lithuania
E-mail: juozas.jarasunas@gmail.com
Žymantas Jagelavičius
Department of Heart and Chest Surgery, Vilnius University, Vilnius, Lithuania
E-mail: zymant@yahoo.com
Abstract. Computed tomography-guided percutaneous core needle biopsy of the lung is an undoubtedly useful and well-established interventional radiological procedure for the diagnosis of indeterminate pulmonary lesions. Complications of percutaneous core needle biopsy, such as pneumothorax and hemoptysis are considered mild and self-resolving, however systemic air embolism is considered a potentially fatal complication. Systemic air embolism occurs when the air enters a pulmonary vein secondary to a percutaneous CT-guided lung biopsy and is expelled into systemic circulation. Systemic air embolism is extremely rare: incidence of clinically apparent SAE is estimated at 0.061–0.17%, while clinically silent systemic air embolism may be as high as 3.8–4.8%. This study reports a case of air embolism in the cerebral arteries that resulted from a complex CT-guided percutaneous core needle biopsy of the lung. The present case highlights the main mechanisms of this pathology, risk factors, importance of complete thoracic CT after procedure, as well as management of rare complications.
Key words: percutaneous core needle biopsy, lung biopsy, systemic air embolism.
Sisteminė oro embolija atlikus kompiuterine tomografija kontroliuojamą perkutaninę plaučio biopsiją: klinikinis atvejis ir literatūros apžvalga
Santrauka. Perkutaninė stulpelinė plaučio biopsija, atliekama kontroliuojant kompiuterine tomografija, neabejotinai naudinga plačiai pripažinta intervencinės radiologijos procedūra, kuria siekiama patikslinti diagnozę esant nenustatytiems plaučių dariniams. Procedūros metu galimos komplikacijos – pneumotoraksas ir hemoptizė, tačiau šios būklės dažniausiai lengvos ir praeina savaime. Rimtesnė, galimai mirtina šios procedūros komplikacija – sisteminė oro embolija. Ji ištinka atlikus perkutaninę plaučio biopsiją, orui patekus į plaučių veną ir nukeliavus į sisteminę kraujotaką. Sisteminė oro embolija, atlikus perkutaninę stulpelinę plaučio biopsiją, ypač reta – kliniškai pasireiškia 0,061–0,17 proc. atvejų (kliniškai nepasireiškianti sisteminė oro embolija sudaro iki 3,8–4,8 proc. atvejų).
Straipsnyje aptariamas klinikinis atvejis, kai pacientei oro embolija smegenų arterijoje nustatyta atlikus sudėtingą, kompiuterine tomografija kontroliuojamą perkutaninę plaučio biopsiją. Aprašomi pagrindiniai numanomi patologijos atsiradimo mechanizmai, aptariami rizikos veiksniai, akcentuojama visapusiško krūtinės ląstos skenavimo, atlikus minėtą procedūrą, svarba, apžvelgiamas šios procedūros retų komplikacijų gydymas.
Reikšminiai žodžiai: perkutaninė stulpelinė biopsija, plaučio biopsija, sisteminė oro embolija.
Received: 2021/08/31. Accepted: 2021/10/05.
Copyright © 2021 Giedrius Ledas, Donatas Jocius, Juozas Jarašūnas, Žymantas Jagelavičius. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Computed tomography (CT) guided percutaneous lung biopsy is well-established diagnostic interventional radiological procedure for the diagnosis of undetermined pulmonary lesions. Pulmonary lesions include diffuse, ground-glass opacities, cystic lesions, subsolid, solid nodules and benign or malignant tumors. Increasing number of CT-guided percutaneous core needle biopsies (PCNB) of the lung leads to rise of case reports of air embolism as a complication of this procedure.
As with any intervention, possible procedure related complications may wary. Minor complications such as pneumothorax and hemoptysis are considered mild and self-resolving, therefore most of the time do not require specific treatment [1]. More severe complications include systemic air embolism (SAE), tumor seeding of the biopsy tract, severe pulmonary hemorrhage, hemothorax, and tension pneumothorax. In this case we present a clinical case of symptomatic SAE occurred in Vilnius University Hospital Santaros Clinics, Lithuania. SAE is extremely rare: incidence of clinically apparent SAE is estimated at 0.061–0.17%, while clinically silent SAE may be as high as 3.8–4.8% [2, 3]. In most cases, the clinical symptoms of SAE present either during procedure, or immediately after it [1, 4].
Case report
62-year-old female with smoking history of 35 pack years was referred to interventional radiology department for a biopsy of the right lung lesion. The lesion was found accidentally during CT investigation before planed aortocoronary bypass surgery. At 6-month follow-up the subsolid lesion remained stable while its suspicious appearance as subsolid nodule with ground glass area at its periphery indicated to perform a biopsy (Figure 1).
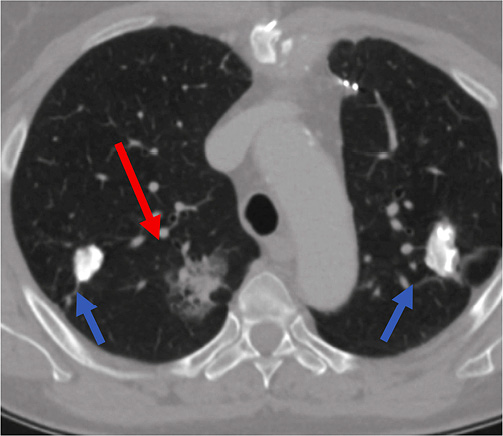
Figure 1. Subsolid lesion of the right lung (red arrow) – suspicious for malignancy. This lesion was the target for lung biopsy. Two additional calcified lesions bilaterally (blue arrows) – most probably related to tuberculosis infection 20 years ago.
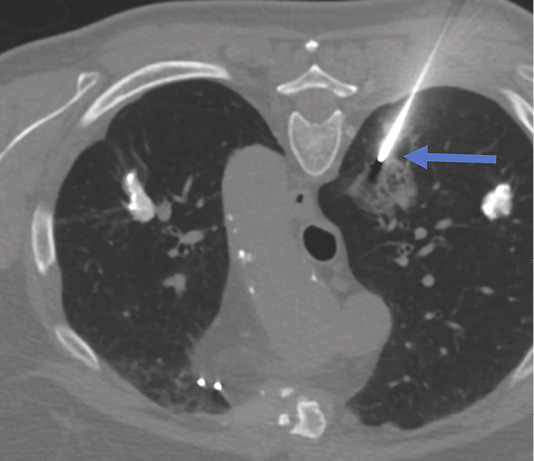
Figure 2. Biopsy of the right lung lesion. Coaxial needle punctured peripheral area of the lesion (blue arrow). Patient is in prone position during the biopsy.
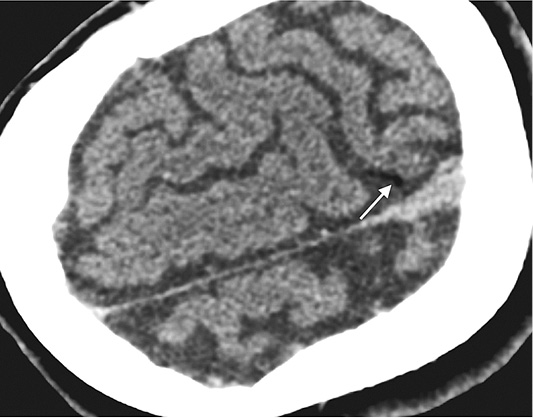
Figure 3. Head CT performed immediately after lung biopsy depicted air (white arrow) in cortical arteries in the left parietal lobe – finding representing air embolism due to lung biopsy.
Lung biopsy procedure was performed under local anesthesia in a prone position and the patient was instructed of shallow breathing. 17 gauge coaxial needle was inserted under CT guidance and proceeded up to the lung needle while lung biopsy was performed with 18 gauge needle with automated biopsy gun (Figure 2) sampling two biopsy specimen cores.
Shortly after second biopsy patient became disorientated, started moving on the CT table. Patient’s language and behavior was also changed, and communication was difficult.
Immediate head CT was performed suspecting neurological pathology. Small air foci were found in peripheral area of left parietal lobe – a clear sign of air embolism (Figure 3). Patient was treated with 100% oxygen.
The next day after the lung biopsy procedure patient felt well, without any neurological disfunction.
Discussion
Pathology mechanism
The consensus on the mechanism of air embolism complicating needle lung biopsy is yet unclear. A number of hypotheses exist, explaining how air can enter systemic blood circulation during CT-guided PCNB [1, 4–6]. One hypothesis is that if the needle tip during the biopsy is placed in a pulmonary vein, and the stylet has been removed, communication between the pulmonary vein and the atmosphere will occur and if the atmospheric pressure exceeds that of the pulmonary vein (the pressure of which is about 10 mmHg), the air is easily blown into the blood. Another hypothesis is that when a needle simultaneously penetrates the alveolus and the pulmonary artery, air is introduced into the pulmonary arterial circulation may reach the pulmonary venous circulation by traversing the pulmonary microvasculature, even in the absence of arteriovenous fistula. Lastly, intra-alveolar or intrabronchial air may be introduced into the pulmonary venous circulation when a bronchial-venous or alveolar-venous fistulas develop when a needle simultaneously traverses an air-containing space (e.g., alveolar, bronchus, cavitary or cystic lesion) and adjacent pulmonary vein, allowing air to enter the pulmonary vein. Factors that elevate intra-alveolar pressure above the venous pressure – coughing, Valsalva maneuver or positive-pressure ventilation – will increase air induction into pulmonary venous circulation. Cases of air embolism without any obvious triggers (e.g. cough, Valsalva maneuver and positive pressure ventilation) could be associated with a silent expiration that forwards air into the pulmonary vein. In the present study, it appeared improbable that air had entered the pulmonary vein directly through the needle, since the hollow of the needle was kept occluded at all times and the patient suspended respiration as instructed during the biopsy. Considering the rapid deterioration of the patient’s condition and air embolism in cerebral arteries found by a CT scan, we hypothesized that the formation of an alveolar-venous or bronchial-venous fistula was the most probable cause of air embolism.
Risk factors
Although the appearance of an air embolism in the left atrium, left ventricle, or systemic circulation during a PCNB of the lung with a subsequent SAE is a rare complication, it can be fatal, therefore all risk factors should be known and taken into account before and during the procedure [2, 3, 6, 7].
Multiple authors agree that main risk factors are prone position of the patient, length of needle path through ventilated lung and number of biopsy samples, level of the lesion above the left atrium [1, 3]. As shown by Monnin-Bares et al., for each additional biopsy sample or for each increase of 5 mm of the length, the probability of air embolism was multiplied by respectively 1.48 (95% CI: 1.01–2.17) and 1.13 (95% CI: 1.02–1.25) [3]. Other risk factors described in the literature include location of the lesion in the lower lobe, endotracheal anesthesia, level of the lesion above that of the left atrium respective to the patient’s position, large number of biopsies, and small CT-slice thickness during the biopsy [3, 8].
For prevention of SAE during PCNB, endotracheal anesthesia should be avoided whenever possible. Patient position should be supine and the tip of the biopsy needle should be within the lesion during the biopsy procedure. Including aerated lung tissue in the biopsy should be avoided whenever is possible.
Importance of complete thoracic CT after procedure
Immediate recognition of SAE has been reported as the main factor to minimize severe complications since specific management of patient can be initiated as soon as possible [1, 9, 10]. However, the incidence rate of air embolism is believed to have been underestimated due to undiagnosed asymptomatic cases. Some studies have suggested a higher incidence based on evaluation of post-procedural thoracic CT images. Hiraki et al. [9] reported clinical incidence rate of 0.4%, based on four cases of air embolism found in complete post-procedural thoracic CT, among 1,010 CT-guided lung biopsies. Of note, Freund et al. [1] showed that the radiological incidence of SAE complicating PCNB was 3.8%, whereas the clinically apparent incidence was 0.49%. The finding that the radiological incidence rate of air embolism is considerably higher than the clinically apparent incidence rate indicates that asymptomatic cases can lead to an overall underdiagnosis of this complication.
Because of possible underdiagnosis, complete thoracic evaluation of patient using CT after the procedure is advised. In 2007, Hiraki et al. described SAE in asymptomatic patients and emphasized the necessity of complete post-procedural thoracic evaluation using CT, in order to visualize SAE [9]. This is supported by fact that for more than 75% of patients, the nodule area can be outside the level of the cardiac cavities [3]. Performing a targeted post procedural CT evaluation only in nodule area on these patients would miss the SAE. Because of this, when evaluating post-procedural computed tomography, operators should use a systemic search pattern to examine for complications. The fact that only 26.1% of the air embolisms were detected during or immediately after the biopsy should draw the conclusion that the radiologist should be more aware of the incidence of SAE during and directly after biopsy and that a meticulous search for intravascular air in the left atrium or left ventricle should be prompted by the radiologist before terminating the procedure. This could prevent the clinical manifestation of SAE, since treatment could be applied without any symptoms present.
Management of complications
As shown in multiple clinical cases, occurrence of SAE can cause a rapid deterioration of patient’s neurological and/or cardiac condition, a prompt management and specialized treatment is required [6, 7]. Rapid treatment could prevent the protean manifestations of SAE, since immediate measures could be initiated, for example Trendelenburg positioning and/or administering pure oxygen. Hyperbaric O2 therapy is a recommended therapy for SAE and has been demonstrated to improve survival rate and neurological recovery. This therapy should be administered as soon as initial stabilization has been achieved. Air embolism may present itself in different ways, depending on location of the arterial air embolus and the volume of the air dispersed into the vessels. When air enters systemic circulation, it disseminated to respective arterial end beds. Most vulnerable systems to end-arterial blockage are the coronary and cerebral systems. Even small volume of air can occlude functional end arteries. As shown in experimental studies, 2 ml of air injected directly into the cerebral circulation is enough to cause fatal effect, while 0.5–1.0 ml of air injected into coronary artery can lead to cardiac arrest. When SAE is asymptomatic, patients should be managed by 100% mask oxygen therapy, electrocardiographic monitoring, respiratory monitoring, prohibition of position changes and repeated CT scans until air has disappeared from blood circulation [11].
Conclusion
Systemic air embolism is an extremely rare complication of percutaneous core needle biopsy of the lung. Although there are several mechanisms described regarding development of this pathology, each case is individual. Known risk factors for this pathology are including aerated lung tissue in the biopsy sample, greater amount of biopsy samples, endotracheal anesthesia, and others. These risk factors should be kept in mind when performing biopsy procedure. As shown by other authors, complete thoracic computed tomography after procedure is undoubtedly valuable and is recommended. Pneumothorax and hemoptysis caused by percutaneous core needle biopsy of the lung can be managed conservatively and should be self-resolving, however, more serious complications such as systemic air embolism should be treated promptly.
References
1. Freund MC, Petersen J, Goder KC, Bunse T, Wiedermann F, Glodny B. Systemic air embolism during percutaneous core needle biopsy of the lung: Frequency and risk factors. BMC Pulm Med 2012; 12.
2. Marchak K, Hong MJ, Schramm KM. Systemic Air Embolism following CT-Guided Percutaneous Core Needle Biopsy of the Lung: Case Report and Review of the Literature. Semin Interv Radiol 2019; 36(2): 68–71.
3. Monnin-Bares V, Chassagnon G, Vernhet-Kovacsik H, Zarqane H, Vanoverschelde J, Picot MC, Bommart S. Systemic air embolism depicted on systematic whole thoracic CT acquisition after percutaneous lung biopsy: Incidence and risk factors. Eur J Radiol 2019; 117: 26–32.
4. Sun C, Bian J, Lai S, Li X. Systemic air embolism as a complication of CT-guided percutaneous core needle lung biopsy: A case report and review of the literature. Exp Ther Med 2015; 10(3): 1157–1160.
5. Mansour A, AbdelRaouf S, Qandeel M, Swaidan M. Acute Coronary Artery Air Embolism Following CT-Guided Lung Biopsy. Cardiovasc Intervent Radiol 2005; 28(1): 131–134.
6. Cheng HM, Chiang KH, Chang PY, Chou YF, Huang HW, Chou ASB, Yen PS. Coronary artery air embolism: a potentially fatal complication of CT-guided percutaneous lung biopsy. Br J Radiol 2010; 83(988): e83–85.
7. Um SJ, Lee SK, Yang DK, Son C, Kim KN, Lee KN, Kim YS. Four cases of a cerebral air embolism complicating a percutaneous transthoracic needle biopsy. Korean J Radiol 2009; 10(1): 81–84.
8. Freund MC, Petersen J, Goder KC, Bunse T, Wiedermann F, Glodny B. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med 2012; 12: 2.
9. Hiraki T, Fujiwara H, Sakurai J, Iguchi T, Gobara H, Tajiri N, Mimura H, Kanazawa S. Nonfatal Systemic Air Embolism Complicating Percutaneous CT-Guided Transthoracic Needle Biopsy: Four Cases From a Single Institution. Chest 2007; 132(2): 684–690.
10. Kok HK, Leong S, Salati U, Torreggiani WC, Govender P. Left Atrial and Systemic Air Embolism after Lung Biopsy: Importance of Treatment Positioning. J Vasc Interv Radiol 2013; 24(10): 1587–1588.
11. Jang H, Rho JY, Suh YJ, Jeong YJ. Asymptomatic systemic air embolism after CT-guided percutaneous transthoracic needle biopsy. Clin Imaging 2019; 53: 49–57.