Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2023, vol. 22(3), pp. 132–143 DOI: https://doi.org/10.15388/LietChirur.2023.22(3).1
Dual Antiplatelet Therapy Regimens and Duration for Intracranial Stenting Procedures: Literature Review
Suggested Protocol for Neuro-interventional Procedure in Lithuania
Milda Paliulytė
Faculty of Medicine, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: milda.paliulyte@gmail.com
Gytis Šustickas
Department of Neurosurgery, Republican Vilnius University Hospital, Vilnius, Lithuania
Faculty of Medicine, Utena University of Applied Sciences, Utena, Lithuania
E-mail: gytis.sustickas@gmail.com
Abstract. Objectives of the study. Our aim was to analyse different antithrombotic drug regimens and duration in intracranial stenting procedures (stent assisted coiling, flow diverter) for unruptured aneurysms and based on the literature review from 2017–2023 to implement dual antiplatelet therapy algorithm for neuro-interventional procedures in Lithuania. Research methods and methodology. A comprehensive literature search of PubMed, BioMed Central, BMJ Journals, EBSCO Publishing, SAGE Journals Online, ScienceDirect, SpringerLink was conducted by two independent readers (MP, GŠ) for studies published from January 2017 to April 2023. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. 23 studies: 6 retrospective cohort, 11 retrospective case-control, 1 prospective cohort, 1 prospective randomized-control, 1 systemic literature review, 3 metanalysis observational studies were identified. Results and conclusions. We found widespread variation in practices even among the same country centres, lending credence to the importance of a future prospective studies of dual antiplatelet drug therapy (DAPT) regimens and duration for the purpose of deriving optimal methods and streamlining tactics. Our suggested algorithm for DAPT in neuro-interventional procedures in Lithuania is provided in Graph 1.
Key words: antiplatelet therapy, aspirin, clopidogrel, dual antiplatelet therapy, flow diverter, pipeline embolization device, prasugrel, PRU – P2Y212 reaction units, stent-assisted coiling, ticagrelol, unruptured aneurysms.
Received: 2023/04/27. Accepted: 2023/06/28.
Copyright © 2023 Milda Paliulytė, Gytis Šustickas. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Endovascular stent – assisted coiling embolization (SACE) or flow diverter (FD) embolization is regarded an effective treatment method for unruptured intracranial aneurysms (UIAs) [1]. FD has been shown to be safe and effective in a number of studies, with a positive safety and efficacy profile in terms of procedure-related complications and aneurysm occlusion rates across different types of aneurysm [2]. The 2.82% risk of thromboembolism during endovascular repair, which can lead to stent occlusion, can be reduced by using antiplatelet (AP) drugs, which work by preventing platelets from aggregating and forming thrombi in the arterial circulation. Aspirin (ASA) and P2Y12 antagonists (clopidogrel (CP), prasugrel (PS), and ticagrelor (TS)) are the discussed examples of AP drugs. There are a number of large-scale randomized control trials on the use of APs after cardiovascular procedures, but for neuro-interventional procedures, there is less data and no established consensus. DAPT consisting of ASA and CP is the most widely used AP regimen [3]. To become effective, CP must first be converted from a prodrug by the hepatic cytochrome enzyme CYP2C19. The effectiveness of medications may be affected by genetic variations in this enzyme or by interactions between different medications. Whilst ASA resistance appears to be 2.1–13.5%, CP resistance is 28–66% and is associated with an increase in thromboembolic events: stent thrombosis, brain ischemia [4]. Since PS and TC are just as effective without this enzyme, there is less individual variation in their dosages. The danger of haemorrhage is another problem associated with AP use, and it can be fatal. Therefore, AP treatments must strike a compromise between avoiding thromboembolic (TE) events associated with FD stents and increasing the risk of bleeding [5].
Results
Table 1 (in page 134) provides a synopsis of the research done between 2017 and 2023 on the optimal AP drug regimen and duration in neuro-interventional radiology.
Discussion
We find widespread variation in practices even among the same country centres, lending credence to the importance of a future prospective studies of drug regimens for the purpose of deriving optimal methods and streamlining tactics.
One possible explanation for these results is the scarcity of randomized controlled trials in the field of neuro-interventional radiology. Even though the degree of evidence is generally poor, certain insights can be extracted from the current literature. Care must also be taken when extrapolating results from cardiology studies as the populations discussed are not equivalent.
Conclusions/recommendations
Taking into consideration the acceptability, DAPT drug prices (CP ≈4 EUR, TC ≈60 EUR, PS ≈500 EUR), dosages (ASA available in 100 mg and 500 mg in Lithuanian market) and common practices of laboratory tests in neuro-interventional radiology in Lithuania as well as summarising the variety of regimens presented in Table 1, we provide recommendations on pre-procedural and post-procedural DAPT regimen and duration in Graph 1.
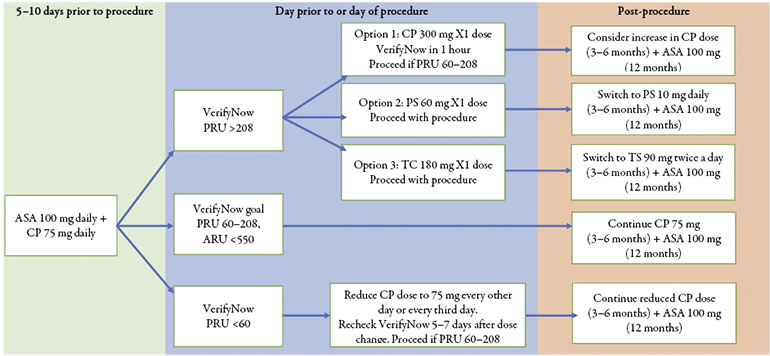
Graph 1. Suggested protocol for DAPT in intracranial stenting procedures of scheduled unruptured aneurysms in Lithuania
Table 1. Summary of recent research findings regarding the use of antiplatelet for neuro-interventions
|
Author (year); study type
|
Type of procedure |
Number of participants (Number of unruptured aneurysms) |
Pathology |
Antiplatelet regime |
Platelet function testing |
Otcomes |
||
|
Pre-operation |
Post-operation |
Efficacy |
Safety |
|||||
|
Prasugrel + AS |
||||||||
|
Pressman et al. [6] (2021); |
PED
|
201
|
Unruptured and ruptured intracranial aneurysms. |
Various regimens:
|
DAPT for 6 months, single ASA long-term.
|
VerifyNow: PRU and ARU were assesed; PRU 60–200. |
1 pt with TIA; 1 pt with in-stent thrombosis; 1 pt with acute stroke. |
69 pts with nuisance bleeds (e.g. easy bruising, bleeding from small cuts, petechiae, ecchymosis). |
|
Xia et al. [7] (2019); metanalysis of observational studies |
Neurointerventional procedures
|
1 394
|
Unruptured intracranial aneurysms.
|
CP vs PS with heterogeneous doses with ASA.
|
VerifyNow: PRU and percentage inhibition of platelet.
|
Lower rate of TE events with PS compared with CP (relative risk [RR] = 0.19, P = 0.0001). |
Equal rates of HH complications (OR = 1.00, p = 1.00).
|
|
|
Cagnazzo et al. [8] (2019); metanalysis of observational studies
|
Neurointerventional procedures
|
1 354 (672)
|
Unruptured intracranial aneurysms.
|
The influence of the intraprocedural UFH administration was not evaluated. Various combinations: PS loading dose: 20 mg vs 30 mg vs 40–60 mg vs 60 mg + ASA 325 mg 1 day before treatment; PS maintenance: 5 mg/day vs 5–10 mg/day vs 10 mg/day. CP loading dose: 75 mg + ASA 100 mg or ASA 325 mg vs 300 mg 5 days before treatment; CP maintenance: 75 mg/day + ASA, 75–100 mg/day vs 75 mg/day + ASA, 325 mg/day. |
|
VerifyNow P2Y12 assay.
|
Low dose PS reduced ischemic events compared with clopidogrel. |
Lower complications rates of low dose PS (20 mg/ |
|
Ticagrelor + ASA |
||||||||
|
Yi et al. [9] (2023); retrospective cohort study |
SACE |
194 (210) |
Unruptured intracranial aneurysms.
|
DAPT with CP 75 mg + ASA 100 mg per day was for at least 5 days. Patients with CP resistance were replaced with TC 90 mg + ASA 100 mg, and the concentration of TC was ensured to reach steady state (about 3 days).
|
ASA 100 mg per day + TC 90 mg twice per day or ASA 100 mg + CP 75 mg per day for 6 months, and then shifted to ASA alone for long-term use. |
Thromboelastography: resistance to ASA was defined as AA inhibition rate <50%, and resistance to CP as ADP inhibition rate <30%.
|
TC seemed to be as effective and safe as CP for SACE in unruptured intracranial aneurysms. Hematocrit and fibrinogen levels were independent risk factors for the incidence of major adverse cardiovascular and cerebrovascular events. |
|
|
Caroff et al. [10] (2021); french comprehensive national survey of 40 neurointerventional surgery centers/ retrospective case-control study |
SACE, Y-confiuration stenting, FD |
Unknown, survey to the centers provided |
Unruptured and ruptured intracranial aneurysms.
|
The most common intravenous (IV) UFH bolus dose was 50 IU/kg – in 59% of centers, 70 IU/kg – in 19%, 60 IU/kg – in 8%, <50 IU/kg – in 8%, 100 IU/kg – in 3% and 80 IU/kg in 3% of centers; followed by a continuous infusion in 72% of centers. UFH was stopped by the end of the procedure in 73% of centers and was prolonged for 24–48 hours in 27%. Only 35% of centers monitored UFH treatment during embolization (with ACT test). DAPT is commonly prescribed, of the centers, 56%, 39%, and 5% used a daily dose of ASA of 75 mg, 160 mg, and 300 mg, respectively. ASA was associated with CP, TC, or PS in 43%, 51%, and 6% of centers. CP resistance was tested for in 94% of the centers using this drug as first-line therapy. When insufficient PUR was found, most centers switched to using TC (87%). |
In cases of SACE, DAPT for 3 or 6 months in 62% and 38% of centers; ASA was prescribed for 6, 12, or 12–24 months in 16%, 57%, and 11% of centers; lifelong ASA – in 16% of centers. In cases of Y-configuration stenting, DAPT for 3 or 6 months in 48% and 52% of centers. ASA for 6, 12, or 12–24 months in 11%, 56%, and 9% of centers; lifelong ASA – in 24% of centers. In cases of FD, DAPT for 3, 6, or 12 months in 39%, 53%, and 8% of centers. ASA for 6, 12, or 12–24 months in 11%, 63%, |
The vasodilator-stimulated phosphoprotein (VASP) test – 43% of centers; VerifyNow – 36% of centers.
|
Not mentioned. |
Not mentioned. |
|
|
|
|
|
All centers using CP reported initiating DAPT at least 5 days before treatment. In contrast, only 26% of centers using TC reported initiating DAPT at least 5 days before treatment; indeed, in 63% of centers it was given the day before. |
and 8% of centers; lifelong aspirin was prescribed in 18% of centers. |
|
|
|
|
Park et al. [11] (2021); retrospective cohort study |
FD, SACE |
201 (233) |
Unruptured intracranial aneurysms.
|
Systematic UFH 500–1000 IU/10 kg of body weight of UFH IV following placement of the femoral/radial sheath, followed by a 1000 IU hourly during the procedure. For the CP group, ASA 81 mg + CP 75 mg once daily for at least 3 days. For the TC group ASA 81 mg once daily + TC 90 mg twice daily for at least 3 days. For patients without any DAPT premedication, |
DAPT was maintained for 3–6 months, followed by discontinuation of CP or TC treatment. ASA monotherapy was maintained indefinitely.
|
Not performed. |
TC appears to be as effective and safe as CP in SACE or FD treatment for unruptured cerebral aneurysms.
|
|
|
Charbonnier et al. [12] (2021); prospective cohort studies |
FD |
413 |
Unruptured and ruptured intracranial aneurysms.
|
ASA + CP; if PRU >208, CP was changed for TC.
|
DAPT for 3 months and then only ASA for 3 additional months.
|
Resistance with PRU. |
Neurological complications after FD implantation were overrepresented by cerebrovascular ischemic events occurring during the peri-operative period, but also in a delayed manner after 1 year. Long-term follow-up is relevant after aneurysm intervention using FD. |
|
|
Li et al. [1] (2021); prospective randomised control study |
PED, FD, SACE |
157 |
Unruptured intracranial aneurysms.
|
DAPT: CP 75 mg + ASA 100 mg; for patients with HPR on CP, CP 75 mg was switched to 1 dose of TC 180 mg before the procedure, followed by 2 daily doses of 90 mg of TC after the procedure. For patients with HPR on ASA, the ASA dose was increased to 200 mg. The treatment adjustment was administered at least 1 day before stenting. |
|
Light transmittance aggregometry: HPR was defined as >50% response to ADP and >20% response to AA. Assessed 1 day prior the procedure and 14 days after, and futher adjustment in DAPT was made.
|
Patients who underwent platelet function monitoring – guided AP therapy before stent placement exhibited an increased clinical benefit related to fewer thromboembolic complications, especially ischemic stroke. |
Although a significant increase in bleeding events was observed in patients with antiplatelet therapy adjustment.
|
|
Mohammaden et al. [13] (2020); retrospective control-case study |
PED
|
24
|
Unruptured and ruptured intracranial aneurysms.
|
During the procedure, an intravenous bolus dose of intravenous UFH 5000 units was administered. Patients who underwent elective FD received 3 days of TC 90 mg twice daily before the procedure followed by the same dose after the procedure. |
|
Not specified. |
No TE complications.
|
1 episode of ruptured aneurysm rebleeding.
|
|
Podlasek et al. [14] (2020); metanalysis of observational studies
|
FD
|
1 005
|
Unruptured and ruptured intracranial aneurysms.
|
CP, PS or TC with ASA. Total of 832 (82.8%) patients received CP + ASA, while 137 (13.6%) patients received TC + ASA, and 36 (3.6%) patients received PS + ASA. Atallah et al. defined clopidogrel hyporesponders as those with platelet inhibition <30% and switched to TC, and to PS if the response was again not satisfactory. Moore et al. defined clopidogrel hyporesponders as those <50% platelet inhibition or PRU >208, and those patients were switched to TC. Adeeb et al. used three different methods to obtain platelet inhibition rate and either switched hyporesponders to TC or gave a loading dose of CP before the procedure. |
|
VerifyNow was performed for all patients only in 3 studies; 2 other studies included selective antiplatelet testing at the discretion of the institution involved. |
Equal TE complications across all 3 groups.
|
TC and PS had lower mortality than CP. Equal HH complications across all 3 groups.
|
|
Soize et al. [4] (2019); retrospective cohort study
|
PED
|
80 (80) (40 pts in CP and 40 pts in TC group)
|
|
UFH bolus of 50 IU/kg followed by a UFH perfusion at 600 IU/kg/day in the perioperative period. 5 days of DAPT (ASA 75 mg + CP 75 mg or TC 90 mg twice |
DAPT for 3 months.
|
Not performed. |
CP group: 3 pts with territorial infarction; 10 pts with >6 small DWI lesions. TC group: 1 pt territorial infarction; 20 pts with >6 small DWI lesions. No stenosis or occlusion of parent vessels noted in either group at 3 months. |
CP group: 2 pts with groin haematomas without active bleeding. No mortality in both groups.
|
|
Narata et al. [3] (2019); retrospective cohort study
|
FD (113), SACE (41)
|
113 (113)
|
Unruptured intracranial aneurysm.
|
2 × loading dose TC 180 mg given evening before and morning of procedure. 2 groups following perioperative UFH dose: group I = 70 U/kg; group II = 50 U/kg. |
TC 90 mg given evening after intervention |
Not performed. |
1.9% ischemic complications.
|
3.9% intracranial HH complications.
|
|
DeGrote et al. [5] (2018); retrospective case-control study |
PED |
29 |
Unruptured and ruptured intracranial aneurysms.
|
TC 90 mg twice daily + ASA 81 mg daily.
|
DAPT (TC + ASA) was continued until |
VerifyNow: if PRU <70 and/or the patient was not appropriately tolerating dual antiplatelet therapy reduce the TC to 60 mg twice daily.
|
Only one ischemic event was observed during the 1-year follow up, and this event occurred after DAPT had been discontinued.
|
No intracranial haemorrhage events were observed.
|
|
Clopidogrel + ASA |
||||||||
|
Al Kasab et al. [15] (2020); retrospective case-control study
|
PED
|
29 (29) |
Unruptured and ruptured intracranial aneurysms.
|
For unruptured aneurysms, patients were bolused with 3,000 units of UFH at procedure onset. Patients with ruptured aneurysms did not receive an UFH bolus. Loaded pre-procedure: DAPT (ASA 325 mg + CP 600 mg). |
DAPT (ASA 325 mg + 75 mg).
|
Not performed. |
4 pts with ischaemic strokes.
|
1 pt with groin haematoma (minor haemorrhage).
|
|
Neyens et al. [16] (2020);
|
PED
|
243 (243) |
Unruptured and ruptured intracranial aneurysms.
|
Anticoagulation was maintained with intermittent UFH bolus fixed at 3000–5000 units, ≈50–100 units/kg to achieve ACT of 200–300 seconds (1.5–2.5 times baseline) for all PED procedures. 24–48 h of loading dose ASA 325–650 mg + CP 600 mg. |
DAPT (ASA and CP) for minimum 6 months. Changed to TC if hyporesponder to CP (48 pts).
|
VerifyNow: compared groups who received personalised antiplatelet therapy according to test results to those who didn’t.
|
7 pts with intraoperative thrombus; 6 pts with non-occlusive thrombus; 3 pts with complete occlusion at day 10, 24 and 20.
|
No difference in pts with HH complications.
|
|
Peret et al. [17] (2020), retrospective case-control study |
FD, SACE |
362 (419) |
Unruptured intracranial aneurysms.
|
The UFH bolus of 5000 IU was followed by a continuous drip (2000 to 2500 IU/h), with the purpose of doubling the baseline ACT. Systemic UFH was prolonged for 12–24 h in all patients. Patients with a weight ≤80 kg received a loading dose of ASA 320 mg + CP 300 mg the day before and on the morning of the procedure. For patients >80 kg, 480 mg of ASA + 450 mg CP. |
DAPT for 3 months (ASA 80 mg + CP 75 mg) and was then seen by a senior neurointerventionalist. ASA 80–100 mg for life after the 3 month appointment. In selected cases, CP 75 mg was also prescribed for 3 extra months. |
Not performed. |
Comparing standard vs high dosing of AP premedication, TE complications rate is low and most of them were clinically silent.
|
HH complications rate was substantial and a significant proportion of them were associated with mortality.
|
|
Bender et al. [18] (2019); |
PED
|
53 (53) |
Unruptured intracranial aneurysms.
|
7 days of DAPT (ASA 325 mg + CP 75 mg).
|
DAPT for life.
|
P2Y12 Reaction unit levels.
|
4 pts with Major ischaemic stroke.
|
Nil pts with intracranial haemorrhages; 1 pt with groin haematoma. |
|
Kim et al. [19] (2018); retrospective cohort study |
SACE |
507 |
Unruptured intracranial aneurysms.
|
DAPT (100 mg ASA + 75 mg CP) was administered for 5 days before the scheduled SACE procedures. On the day of the procedure, DAPT was administered in the morning and systemic UFH was administered after femoral sheath placement in the operating room.
|
DAPT duration (short-term, <9 months; long-term, ≥9 months) to evaluate delayed TE events.
|
P2Y12 response testing routinely only starting from 2015.
|
Compared with short-term DAPT, long-term DAPT can delay the occurrence of delayed TEs but does not lower their incidence. Most events occurred within 1 month of switching from DAPT to single-antiplatelet therapy, regardless of DAPT duration. |
|
|
Cagnazzo et al. [20] (2018); retrospective case-control study
|
12 aneurysms with PED; 3 aneurysms with Silk; 2 aneurysms with FRED
|
15 (17) |
Unruptured and ruptured intracranial aneurysms.
|
Concurrent with the procedure, intravenous UFH was performed (activated clotting time maintained above 250 seconds). Minimum 5 days of DAPT (ASA 75 mg + CP 75 mg). |
DAPT for minimum 6 months, after clinical and radiological evaluation switched to ASA for 1 year.
|
VerifyNow
|
2 pts with stent thrombosis (1 occurring 12 h post intervention, 1 occurring 10 days after due to discontinuation of DAPT); 1 pt with small basal ganglia infarct due to insufficient platelet inhibition 24 h after treatment. |
Nil HH complications.
|
|
Patel et al. [21] (2017);
|
PED
|
100 (100) |
|
Following groin puncture, patients received boluses of IV UFH with a goal ACT of 2–2.5 times the baseline value for the duration of the procedure. 1–4 weeks of DAPT (ASA 325 mg + CP 75 mg). CP dose is adjusted depending on assay.
|
DAPT for 6–12 months.
|
P2Y12 assay; a goal P2Y12 reaction unit value 60–230.
|
5 pts developed acute thrombus formation intraoperatively, requiring Abciximab loading dose. 3 month follow-up showed gross patency of PED in all patients. |
1 pt with small intraparenchymal haemorrhage.
|
|
Peschillo et al. [22] (2017); retrospective case-control study
|
PED, Silk
|
26 (26) |
Unruptured and ruptured intracranial aneurysms.
|
3–10 days of DAPT (ASA 100–300 mg + CP 75 mg).
|
DAPT for 3–12 months. 5 groups used ASA + CP; 1 group used ASA + TC; 1 group used ASA + tirofiban + CP.
|
Not performed. |
1 pt with intraoperative TE; 1 pt with stent occlusion at 3 months; 4 pts had FD deploy complication causing steno-occlusion intra-operatively; 1 pt with TE intraoperatively. |
2 pts died, 1 due to cardiac complications and the other unknown cause.
|
|
Texakalidis et al. [23] (2017); systemic literature review |
PED |
1 556 (1 382) |
Unruptured and ruptured intracranial aneurysms.
|
ASA 300–325 mg (2–14 days) + CP 75 mg (3 to more than 10 days) before elective PED in 61.7% of patients. ASA 81 mg + CP 75 mg for 5–10 days were used in 27% of patients. In 6.3% of them, ASA 100–150 mg + CP 75 mg for 5 days was used.
|
ASA >6 months and CP 3–12 months was the regimen of choice for 93% of patients.
|
The VerifyNow: PRU in 9 out of 13 studies; PRU + ARU in 4 out of 13. |
There was no statistically demonstrable relationship between TE events in patients who received low dose (81–150 mg) pre-PED ASA versus high dose (300–325 mg) pre-PED ASA (9.4% vs 8.1%, p = 0.32). |
In patients who received low versus high dose of pre-PED ASA, HH events occurred at 0.7% and 3.3% respectively; however, no statistical significance was achieved (p = 0.053). |
|
Adeeb et al. [24] (2017); retrospective case-control study |
PED |
402 |
Unruptured and ruptured intracranial aneurysms.
|
UFH 3000–5000 U bolus at the beginning of the procedure, with hourly dosing of 1000 U, ACT target 250–300. ASA 325 mg + CP 75 mg daily for 3–14 days before the intervention to achieve 50–60% platelet inhibition. The last dose of CP was given the morning of platelet function testing. If a patient was a CPl non-responder: to continue on same dose CP vs one-time 600 mg CP boost within 24 hours pre-procedure vs switch to TC. |
DAPT for at least 3 months and ASA indefinitely thereafter.
|
The VerifyNow: PRU >208 CP non-responders.
|
CP non-responders experienced a significantly higher rate of TE complications when compared with CP responders (17.4% vs 5.6%; p = 0.0002).
|
There was no significant difference in the HH complications between groups.
|
AA – arachidonic acid, ACT – activated clotting time, AP – antiplatelet, ARU – Aspirin Reaction Units, ASA – acetylsalicylic acid (aspirin), CP – clopidogrel, DAPT – dual antiplatelet therapy, DWI – diffusion weighted imaging, FD – flow diverter, FRED – Flow Re-direction Endoluminal Device, HH – haemorrhagic, HPR – high on treatment platelet reactivity, PED – Pipeline Embolization Device, PRU – P2Y212 ReactionUnits, PS – prasugrel, SACE – Stent-assisted coil embolization, TC – ticagrelol, TIA – transient ischaemic attack, TE – thromboembolic, UFH – unfractioned heparin.
References
1. Li W, Zhu W, Wang A, Zhang G, Zhang Y, Wang K, Zhang Y, Wang C, Zhang L, Zhao H, Wang P, Chen K, Liu J, Yang X. Effect of Adjusted Antiplatelet Therapy on Preventing Ischemic Events After Stenting for Intracranial Aneurysms. Stroke 2021; 52(12): 3815–3825.
2. Leung AL, Li V, de Villiers L, Hattingh L. A Comparison of Antiplatelet Therapy During the Peri- and Post-Operative Periods Following Flow-Diverting Stent Insertion for Unruptured Intracranial Aneurysms: A Systematic Review. Interv Neuroradiol 2023.
3. Narata AP, Amelot A, Bibi R, Herbreteau D, Angoulvant D, Gruel Y, Janot K. Dual Antiplatelet Therapy Combining Aspirin and Ticagrelor for Intracranial Stenting Procedures: A Retrospective Single Center Study of 154 Consecutive Patients With Unruptured Aneurysms. Neurosurgery 2019; 84(1): 77–83.
4. Soize S, Foussier C, Manceau PF, Litré CF, Backchine S, Gawlitza M, Pierot L. Comparison of Two Preventive Dual Antiplatelet Regimens for Unruptured Intracranial Aneurysm Embolization with Flow Diverter/Disrupter: A Matched-Cohort Study Comparing Clopidogrel with Ticagrelor. J Neuroradiol 2019; 46(6): 378–383.
5. DeGrote JR, Olafson EM, Drofa A, Kouznetzov E, Manchak M, Leedahl ND, Leedahl DD. Ticagrelor and Acetylsalicylic Acid After Placement of Pipeline Embolization Device for Cerebral Aneurysm: A Case Series. Can J Hosp Pharm 2018; 71(6): 349–355.
6. Pressman E, De la Garza CA, Chin F, Fishbein J, Waqas M, Siddiqui A, Snyder K, Davies JM, Levy E, Kan P, Ren Z, Mokin M. Nuisance Bleeding Complications in Patients with Cerebral Aneurysm Treated with Pipeline Embolization Device. J Neurointerv Surg 2021; 13(3): 247–250.
7. Xia P, He C, Chen L, Zou L, Sun S, Cui P, Wang W. Efficacy and Safety of Prasugrel Therapy for Intracranial Aneurysms with Endovascular Treatment: A Meta-Analysis. J Neurol Sci 2019; 397: 174–178.
8. Cagnazzo F, Perrini P, Lefevre PH, Gascou G, Dargazanli C, Riquelme C, Derraz I, di Carlo D, Bonafe A, Costalat V. Comparison of Prasugrel and Clopidogrel Used as Antiplatelet Medication for Endovascular Treatment of Unruptured Intracranial Aneurysms: A Meta-Analysis. AJNR Am J Neuroradiol 2019; 40(4): 681–686.
9. Yi MM, Do HP, Li YC, Wang R, Zhuang Z, Xu MM, Liu T, Shao TF, Ding LP, Ge WH. Ticagrelor versus Clopidogrel in the Dual Antiplatelet Regimen for Unruptured Intracranial Aneurysm Treated with Stent-Assisted Coil Embolization: A Single-Center Cohort Study. World Neurosurg 2023; 170: e755–e765.
10. Caroff J, Aubert L, Lavenu-Bombled C, Figueiredo S, Habchi K, Cortese J, Eugene F, Ognard J, Tahon F, Forestier G, Ifergan H, Zhu F, Hak JF, Reyre A, Laubacher M, Traore A, Desilles JP, Derraz I, Moreno R, Bintner M, Charbonnier G, Le Bras A, Veunac L, Gariel F, Redjem H, Sedat J, Tessier G, Dumas V, Gauberti M, Chivot C, Consoli A, Bricout N, Tuilier T, Guedon A, Pop R, Thouant P, Bellanger G, Zannoni R, Soize S, Richter JS, Heck O, Mihalea C, Burel J, Girot JB, Shotar E, Gazzola S, Boulouis G, Kerleroux B; JENI Research Collaboration. Antithrombotic Therapies for Neurointerventional Surgery: A 2021 French Comprehensive National Survey. J Neurointerv Surg 2023; 15(4): 402–407.
11. Park KY, Ozaki T, Kostynskyy A, Kortman H, Hilario A, Nicholson P, Agid R, Krings T, Pereira VM. Ticagrelor versus Clopidogrel in the Dual Antiplatelet Regimen for Intracranial Stenting or Flow-Diverter Treatment for Unruptured Cerebral Aneurysms: A Single-Center Cohort Study. AJNR Am J Neuroradiol 2021; 42(9): 1638–1644.
12. Charbonnier G, Desilles JP, Escalard S, Maier B, Ciccio G, Smajda S, Fahed R, Delvoye F, Redjem H, Blanc R, Piotin M, Mazighi M. Timing and Spectrum of Neurological Complications After Flow Diverter Implantation for Intracranial Aneurysms. Front Neurol 2021; 12: 590383.
13. Mohammaden MH, English SW, Stapleton CJ, Khedr E, Shoyb A, Hegazy A, Elbassiouny A. Safety and Efficacy of Ticagrelor as Single Antiplatelet Therapy in Prevention of Thromboembolic Complications Associated with the Pipeline Embolization Device (PED): Multicenter Experience. J Neurointerv Surg 2020; 12(11): 1113–1116.
14. Podlasek A, Al Sultan AA, Assis Z, Kashani N, Goyal M, Almekhlafi MA. Outcome of Intracranial Flow Diversion According to the Antiplatelet Regimen Used: A Systematic Review and Meta-Analysis. J Neurointerv Surg 2020; 12(2): 148–155.
15. Al Kasab S, Guerrero WR, Nakagawa D, Samaniego EA, Ortega-Gutierrez S, Hasan D. Safety and Efficacy of the Pipeline Embolization Device Use in the Outside Circle of Willis Located Intracranial Aneurysms: A Single-Center Experience. Interv Neurol 2020; 8(2–6): 83–91.
16. Neyens R, Donaldson C, Andrews C, Kellogg R, Spiotta A. Platelet Function Testing with a VerifyNow-Directed Personalized Antiplatelet Strategy and Associated Rates of Thromboembolic Complications After Pipeline Embolization for Complex Cerebral Aneurysms. World Neurosurg 2020; 138: e674–e682.
17. Peret A, Mine B, Bonnet T, Ligot N, Bouziotis J, Lubicz B. Safety and Efficacy of a Pre-Treatment Antiplatelet Regimen of Unruptured Intracranial Aneurysms: A Single-Center Experience. Neuroradiology 2020; 62(8): 1029–1041.
18. Bender MT, Colby GP, Jiang B, Lin LM, Campos JK, Xu R, Westbroek EM, Vo CD, Zarrin DA, Caplan JM, Huang J, Tamargo RJ, Coon AL. Flow Diversion of Posterior Circulation Cerebral Aeurysms: A Single-Institution Series of 59 Cases. Neurosurgery 2019; 84(1): 206–216.
19. Kim T, Kim CH, Kang SH, Ban SP, Kwon OK. Relevance of Antiplatelet Therapy Duration After Stent-Assisted Coil Embolization for Unruptured Intracranial Aneurysms. World Neurosurg 2018; 116: e699–e708.
20. Cagnazzo F, Cappucci M, Dargazanli C, Lefevre PH, Gascou G, Riquelme C, Bonafe A, Costalat V. Treatment of Distal Anterior Cerebral Artery Aneurysms with Flow-Diverter Stents: A Single-Center Experience. AJNR Am J Neuroradiol 2018; 39(6): 1100–1106.
21. Patel A, Miller TR, Shivashankar R, Jindal G, Gandhi D. Early Angiographic Signs of Acute Thrombus Formation Following Cerebral Aneurysm Treatment with the Pipeline Embolization Device. J Neurointerv Surg 2017; 9(11): 1125–1130.
22. Peschillo S, Caporlingua A, Resta MC, Peluso JPP, Burdi N, Sourour N, Diana F, Guidetti G, Clarençon F, Bloemsma GC, Di Maria F, Donatelli M, Resta M. Endovascular Treatment of Large and Giant Carotid Aneurysms with Flow-Diverter Stents Alone or in Combination with Coils: A Multicenter Experience and Long-Term Follow-up. Oper Neurosurg (Hagerstown) 2017; 13(4): 492–502.
23. Texakalidis P, Bekelis K, Atallah E, Tjoumakaris S, Rosenwasser RH, Jabbour P. Flow Diversion with the Pipeline Embolization Device for Patients with Intracranial Aneurysms and Antiplatelet Therapy: A Systematic Literature Review. Clin Neurol Neurosurg 2017; 161: 78–87.
24. Adeeb N, Griessenauer CJ, Foreman PM, Moore JM, Shallwani H, Motiei-Langroudi R, Alturki A, Siddiqui AH, Levy EI, Harrigan MR, Ogilvy CS, Thomas AJ. Use of Platelet Function Testing Before Pipeline Embolization Device Placement: A Multicenter Cohort Study. Stroke 2017; 48(5): 1322–1330.