Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2025, vol. 24(1), pp. 17–26 DOI: https://doi.org/10.15388/LietChirur.2025.24(1).2
Mammary Hamartoma: A Narrative Review of a Rare Breast Disorder
Sajad Ahmad Salati
Department of Surgery, College of Medicine, Qassim University, Saudi Arabia
E-mail: docsajad@gmail.com
ORCID: https://orcid.org/0000-0003-2998-7542
Faiza Riaz Malik
Department of Surgery, Qassim University Medical City, Saudi Arabia
E-mail: faiza.r.malik@hotmail.com
ORCID: https://orcid.org/0009-0007-3410-6021
https://ror.org/01wsfe280
Abstract. Mammary hamartomas are rare, benign tumors that primarily affect women in their middle years. Tumor cells originating from native breast tissues are arranged in a disorderly way, which is their defining characteristic. In recent years, there has been a growing number of reports about them. A correlation between clinical, radiological, and histological findings is necessary to make the diagnosis. To raise awareness of this rare disorder and encourage medical professionals to take it into account when making a differential diagnosis of breast lumps, this review paper provides a brief overview of the epidemiology, pathogenesis, clinical presentation, investigations, and therapy of MH.
Keywords: mammary hamartoma, breast cancer, surgical excision, cancer, lobule, adipose tissue, recurrence.
Received: 2024-12-14. Accepted: 2025-01-18.
Copyright © 2025 Sajad Ahmad Salati, Faiza Riaz Malik. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Mammary hamartomas (MH) are rare benign tumors. The tumor cells are arranged in a disorderly fashion and originate from the native cells of the mammary ducts, lobules, and connective tissue stroma [1–2]. MH is defined by the World Health Organization (WHO) as “a well-demarcated, generally encapsulated mass, composed of all components of breast tissue”.
In 1928, Pryn made the first identification of this lesion and referred to it as a “mastoma”. Individual cases were later reported in the literature as lipofibroadenoma, adenohibernoma, fibroadenolipoma [3–4], and adenolipoma [5]. The term “mammary hamartoma” was first coined by Arrigoni et al. in 1971, when they described it as a well-defined mass formed by abnormal cells intermixed with normal breast tissue [6]. In 1981, breast hamartoma was included in the World Health Organization’s classification system of diseases [7].
This review article briefly summarizes the epidemiology, pathophysiology, clinical presentation, investigations, and treatment of MH to increase awareness of this rare disorder and encourage medical professionals to consider it when making a differential diagnosis of breast masses.
Methods and materials
A thorough search was carried out in PubMed, Google Scholar, and ResearchGate using the keywords “breast hamartoma”, “mammary hamartoma”, “benign breast disorders”. The reference lists of the articles retrieved were examined to identify additional relevant sources. The literature from 2010–2024 was given priority, and earlier sources were only used when they contained some historical context.
Epidemiology
Mammary hamartomas account for approximately 4–8% of all benign breast lesions [8–9]. The predominant demographic of patients with MH consists of females in their forties; however, the age of onset may range from the thirties to the sixties [10]. Male MH have also been documented, and they account for about 0.12%–0.24% of all male breast tumors [11].
MH is frequently underdiagnosed, which leads to underreporting, primarily due to lack of awareness of its distinctive clinical and histological characteristics [12]. However, in recent years, the diagnosis of this entity has grown with the growing use of diagnostic methods for breast cancers, such as core needle biopsy, fine needle aspiration cytology, ultrasonography, and mammography [13–15]. Studies have been conducted to elucidate potential risk factors, and it has been found that there is no association with ethnic origin or dietary habits [16].
Etiopathogenesis
The etiopathogenesis of MH is not clear [15], but it is believed to be the result of dysgenesis rather than a true neoplastic process. Some studies have linked the development of MH to female sex steroid hormones. Herbert et al. (17) conducted immunohistochemical studies in excised specimens of MH in 24 cases. They detected estrogen (ER) and progesterone (PR) receptor positivity in epithelial cells as well as in the stromal cells in all the specimens. There was no c-erbB-2 protein overexpression nor p53 expression. In most cases, Ki67 showed 2–3% positivity in epithelial cells and not in stromal cells. Furthermore, the origin of smooth muscle in myoid hamartomas is unclear; nevertheless, it may originate from arteries, the nipples, undifferentiated breast stromal tissue, or myoepithelial cells [9]. Kajo et al. [17], based on hormone receptor expression of the cells, hypothesized smooth muscle metaplasia of the hormonally responsive breast stromal cells to be the origin. MH has been observed to enlarge during pregnancy and lactation, suggesting that endocrine variables and hormones may contribute to the formation of these tumors.
Histopathology
Gross inspection of the specimen retrieved by excision biopsy reveals a soft to firm, smooth-surfaced, nodular mass enclosed in a false capsule. On cut sections, the fibrotic stroma with varying fatty regions gives rise to a rubbery/fleshy surface with a grey or yellow colour [18]. Clefts and fronds are not visible.
Microscopically, the lesion shows fibrotic stroma extending between individual breast lobules and adipose tissue in varying proportions with disorganized architecture (Figure 1). Adipose tissue is present in more than 90% of the lesions, and its volume generally accounts for 10–30% of the lesion volume [18]. The lesions may have variable amounts of smooth muscle, cartilage, or pseudoangiomatous stromal hyperplasia (PASH).
The presence of PASH within the MH lesion has been described as a constant entity, with the reported incidence varying from a high 93.6% [19] to a low 16–20% [20]. In a series by Tse et al. [18], PASH was found in 32% of the specimens. MH lesions with prominent myoid changes represented by extensive foci of spindle cells are termed as myoid hamartomas [17].
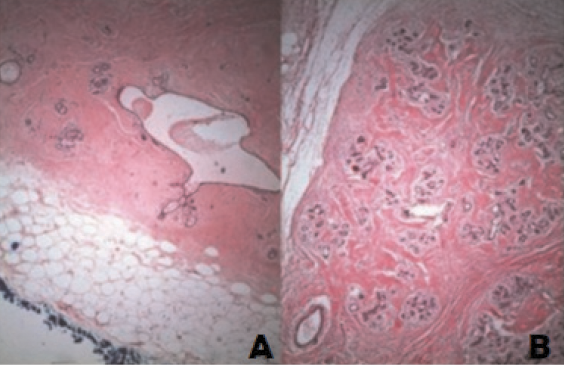
Figure 1 (A) and (B). Well-circumscribed mass with normal terminal ductal lobular unit, fat and hyalinized stroma, Haematoxylin Eosin (H&E), X 640. Image credits: Sevim Y, Kocaay AF, Eker T, Celasin H, Karabork A, Erden E, Genc V. Breast hamartoma: a clinicopathologic analysis of 27 cases and a literature review. Clinics (Sao Paulo) 2014; 69(8): 515–523. DOI: 10.6061/clinics/2014(08)03. Reused under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/).
Needle core biopsies and fine needle aspiration cytology are generally insufficient, inconclusive, or non-specific [17–18]. Alran et al. [19], however, found that if intralesional location is ascertained with imaging, the core needle biopsy can prove to be effective in diagnosing MH if the presence of stromal changes, PASH, interlobular distribution of CD34-positive fibroblasts, HMGA2, and hormonal receptor stromal expression are considered.
Clinical presentation
Clinically, MH typically manifests as a painless mass. However, they may also present as breast pain, breast asymmetry, or else be incidentally detected during routine screening mammography or other breast imaging modalities [2]. Most hamartomas grow somewhat slowly. Nonetheless, some lesions, which are particularly common in pregnant and breastfeeding women, rapidly enlarge.
On physical examination, clinically apparent MH appear as soft, non-tender, globular, or oval nodules with smooth surfaces that are mobile against their surrounding tissues [21]. Neglected lesions may result in compressive symptoms with skin necrosis, ulceration, and lymphedema.
MH ranges in size from tiny lumps to tumor-like structures that can fill nearly the whole breast and have a diameter of 10–12 cm. Subclinical hamartomas, which cannot be palpated, account for up to 60% of all cases [21].
There are a few reports of MH in the literature with greater dimensions (above 10 cm) and termed as giant MH. Ahire et al. [22] reported a 48-year-old female who had presented with a progressively enlarging right breast lump over eight years. After evaluation, a well-encapsulated giant MH, measuring 29x25x11.5 cm and weighing 6 kg, was excised. The lesion had no malignant features. Rumpf et al. [23] presented a rare case of a 36-year-old woman with a giant MH measuring 15 cm and weighing 700 grams. She had been treated for breast cancer in the contralateral breast one year. Palo and Agrawal have reported a case of a 38-year-old woman with firm, movable MH in her right breast that measured 21 by 15 cm and clinically resembled a phyllodes tumor [24].
Imaging
MH mostly presents characteristic radiological appearances; however, atypical MH can mimic other benign and malignant lesions [24]. The radiological modalities commonly used for diagnosis are as follows.
Mammography
On mammography, MH is typically seen as an inhomogeneous oval or round formation, with radio-opaque and radiotransparent areas that reflect the presence of tissues of different densities (Figure 2). The lesion bears a thin, well-defined radiopaque border (pseudocapsule). The presence of different densities within the pseudocapsule tends to create a pathognomonic impression of a “slice of salami” [2, 25] or a bull’s eye [2] or a “breast within a breast” [26].
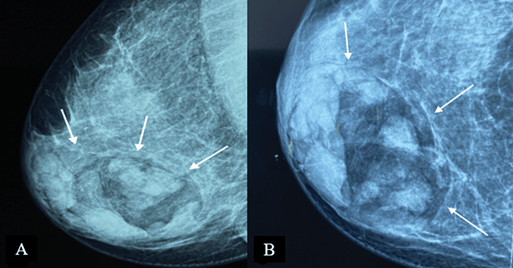
Figure 2. Digital mammography: medio-lateral oblique (A) and cranio-caudal (B) projections of the right breast – a large, well-defined mass containing radiolucent (fat) and radiopaque (soft tissue) densities can be seen. It is surrounded by a thin radiopaque capsule, presenting a “breast within a breast” appearance. Image credits: El Yousfi Z, El Mansoury FZ, El Bakkari A, Omor Y, Latib R. Breast hamartoma with synchronous contralateral breast cancer: a case report. Cureus 2024; 16(8): e66534. DOI: 10.7759/cureus.66534. Reused under the terms of the Creative Commons Attribution License CC-BY 4.0. (https://creativecommons.org/licenses/by/4.0/deed.en).
Ultrasound
On ultrasound, the appearance of MH differs widely owing to the marked variability in the fatty and fibrous tissue constituents. They generally appear as solid, well-circumscribed, oval formations with heterogeneous echogenicity [24], aligned parallel to the skin plane (Figure 3). The lesion typically lacks hypervascularization on colour Doppler imaging and shows an echogenic or echolucent halo with posterior strengthening [2]. Incomplete pseudocapsule and tiny size with minimal fat content might make diagnosis challenging [8].
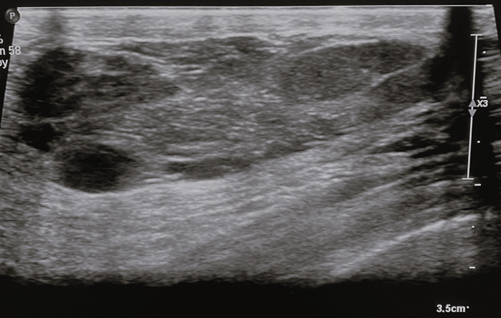
Figure 3. Breast ultrasound – breast ultrasound shows a tissue formation with the long axis parallel to the cutaneous plane, well-circumscribed, heterogeneous, with hypoechoic with hyperechoic trabeculae and posterior acoustic enhancement. Image credits: El Yousfi Z, El Mansoury FZ, El Bakkari A, Omor Y, Latib R. Breast hamartoma with synchronous contralateral breast cancer: a case report. Cureus 2024; 16(8): e66534. DOI: 10.7759/cureus.66534. Reused under the terms of the Creative Commons Attribution License CC-BY 4.0. (https://creativecommons.org/licenses/by/4.0/deed.en).
Magnetic resonance imaging (MRI)
MRI can be useful in establishing a diagnosis if suspicious features are noted on mammography or ultrasound [27]. Otherwise, MRI is not generally indicated for typical MH. On T1- and T2-weighted sequences (Figure 4), hamartomas appear as heterogeneously intense masses with glandular and fatty tissue components and a thin capsule [24]. Once the contrast media has been administered, MH exhibits a gradual, progressive enhancement with a type I time/intensity curve [28].
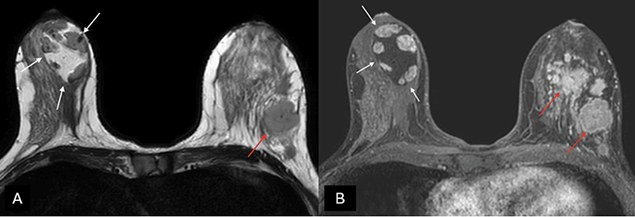
Figure 4. (A) Axial T2-weighted images and (B) T1-weighted fat-suppressed enhanced magnetic resonance imaging (MRI) – a capsulated, large-sized hamartoma can be seen located in the outer quadrants of the right breast, exhibiting a signal identical to normal mammary gland tissue, creating a “breast within a breast” appearance (white arrows). There are multiple masses in the contralateral breast with suspicious morphology, associated with a known invasive ductal carcinoma (red arrows). Image credits: El Yousfi Z, El Mansoury FZ, El Bakkari A, Omor Y, Latib R. Breast hamartoma with synchronous contralateral breast cancer: a case report. Cureus 2024; 16(8): e66534. DOI: 10.7759/cureus.66534. Reused under the terms of the Creative Commons Attribution License CC-BY 4.0. (https://creativecommons.org/licenses/by/4.0/deed.en).
Differential diagnosis
The differential diagnosis includes a range of disorders [18] including:
Fibroadenomatoid mastopathy. In fibroadenomatoid mastopathy (sclerosing lobular hyperplasia), the lesion is a well-defined, spherical mass with expanded lobules [29]. It resembles MH because of the interlobular stromal sclerosis but can be distinguished by its frequent connection with fibroadenoma and the lack of a fatty component in the stroma.
Lipoma. A lipoma is a benign tumor made of fat tissue. It is movable and painless like MH. It closely resembles MH with a higher proportion of fatty components.
Liponecrosis. Liponecrosis is a benign inflammatory process that occurs when there is saponification of local fat. It may manifest as an oil cyst or else as a spiculated lesion, depending on the degree of fibrosis [29]. Its association with the history of surgery or other types of trauma facilitates the diagnosis [18].
Fibroadenoma. MH that contain relatively high amounts of fibrous stroma tend to be homogeneously dense, and thus it may be challenging to differentiate from fibroadenomas.
Cystosarcoma phyllodes. Cystosarcoma Phyllodes is a rare fibroepithelial neoplasm [30] that closely mimics giant MH with a greater amount of fibrous tissue. The quick growth and the presence of an epithelial component help in diagnosis [18].
Relationship of mammary hamartoma with breast cancer
MH is often not regarded as a premalignant lesion; nonetheless, given their glandular breast tissue, the malignant transition is possible, like normal breast tissue. As a result, case-by-case examination and follow-up should be done in an individualized manner [31]. Choi and Ko [32], in a review of the literature, described 15 cases of cancer associated with MH. In most cases, the diagnosis was made after detection of suspicious features on imaging, which included clusters of microcalcifications, pleomorphic microcalcifications, and spiculated opacities on mammography and irregular hypoechoic lesions on ultrasonography. In two cases, coexisting cancer was discovered unexpectedly on histopathological analysis of the excised specimen.
Soltani et al. [33] very recently reported a 46-year-old woman in whom a long-standing breast lump appeared highly suspicious on imaging. On mammogram, the lump was approximately 4.5×4×3 cm with an irregular shape and spiculated margins associated with coarse heterogeneous microcalcifications. MRI confirmed the presence of an oval-shaped circumscribed mass of predominant internal adipose intensity (non-enhancing) containing an internal irregular spiculated mass of heterogenous T2 hypo-intensity with heterogenous enhancement and medium/plateau kinetics. A BIRADS score of 5 was reported in both imaging modalities. However, the histopathological analysis of the surgically excised specimen ruled out cancer and established the diagnosis of MH. Yousfi et al. [26] reported a 37-year-old female who presented with a large palpable mass in the right breast, and on imaging, was found to have synchronous contralateral breast cancer.
Kurodo et al. [34] reported a case of a 53-year-old woman in whom the MH was confirmed on histological examination of the breast lesion, and cancer with foci of microinvasion was observed in the lobules of the MH lesion concomitant with the intraductal spread of lobular carcinoma. Ruiz-Tovar et al. [35] reported a case of a 35-year-old woman in whom mammography had shown the features of a typical hamartoma with suspicious microcalcifications, and histopathological examination of the specimen revealed infiltrating ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS) associated with it. Choi et al. [32] reported a case of a 70-year-old woman who was treated conservatively for MH, and six years later reported back with mammogram changes around the mass, which turned out to be an invasive ductal carcinoma.
Mammary hamartoma in men
Despite the possibility that males may also experience breast diseases, breast hamartomas are primarily thought to be a female-specific condition, and only five cases have been reported in the peer-reviewed literature. According to Phan et al. [11], a 41-year-old male had a left breast lump for 15 years that was around 10 cm from the nipple. The lump has gotten bigger and tender. A mammogram showed a 30×9 mm tumor in the upper outer quadrant of the left breast, surrounded by clustered coarse heterogeneous calcifications and microlobulated borders. In line with hamartoma, ultrasound-guided core biopsies showed smooth muscle bundles together with fibrous stromal and adipose tissue. The patient received conservative treatment, and at his follow-up check a year later, he was still asymptomatic and had no new breast lumps.
Li et al. [36] reported a 30-year-old man who had presented with a one-month history of a painless mass in his right breast. A lesion of around 2.0 cm by 2.0 cm was discovered in the right breast by ultrasound imaging and mammography. It was provisionally diagnosed as an adenoma fibrosum or a lipomyoma. Surgical excision was conducted, and the histopathological examination confirmed the diagnosis of mammary hamartoma. There was no recurrence at one year.
Mammary hamartoma in childhood and adolescence
A few cases of MH in children and adolescents have been reported in the literature. Chang et al. [37] retrospectively analyzed patient records at the Massachusetts General Hospital, Boston, and identified 7 cases of breast hamartomas in patients less than 18 years of age over a 17-year period. In 5 cases, surgical excision was undertaken, and in 2 cases, core needle biopsy for diagnosis was the only intervention. Recurrence was recorded in one case 9 months after surgical excision.
Gupta et al. [38] reported a case of a 10×10 cm MH in the right breast of a 13-year-old boy. The mass spread into the thoracic cavity with the help of pseudopodia-like projections through the 3rd and 4th intercostal spaces without the evidence of any rib destruction. FNAC analysis was not helpful in clinching the diagnosis. Management involved complete surgical excision, along with anterior portions of the 3rd and 4th ribs, while preserving the nipple-areola complex. Histological examination of the specimen confirmed it to be a hamartoma, and there was no recurrence at 14 months.
Impact on quality of life
If left untreated, these lesions may enlarge and result in breast asymmetry, which can have a lasting psychosocial impact that may lead to low self-esteem and interfere with interpersonal relationships, particularly if the lesions are not initially identified during adolescence or early adulthood when relationship dynamics are being established [39].
Management
Treatment is based on the patient’s clinical presentation, and options range from reassurance to surgical excision. Surgical excision is the most common form of management. It is essential to ensure that the lesion is excised with a clear surgical margin due to the possibility of recurrence and, in rare instances, possible malignancy foci inside the lesion [6]. Tazeoglu [6] presented a series of 39 cases, all of whom (100%) were treated surgically. Lumpectomy and simple mastectomy were performed in 37 (94.9%) and 2 (5.1%) cases, respectively. In 6 out of 37 (16.2%), lumpectomy was undertaken using radio-guided stereotactic marking because of the small sizes of the masses. Mastectomy was selected as an option in two patients due to the high mass/breast volume ratio.
Ultrasound (US)-guided vacuum-assisted breast biopsy (VABB) is an alternative non-surgical approach that has been employed in recent years. Hu et al. [40] prospectively treated 31 cases of MH with this approach. Complete excision rate was 96.8% (30/31), and recurrence was seen in 1 (3.2%) case one year after the procedure. Complications encountered were of minor nature, which included pain (22.6%), hematomas (9.7%), and ecchymosis (3.2%).
Conclusion
Mammary hamartoma is a rare, benign breast lesion that is being increasingly detected in recent times because of the growing use of breast imaging for breast disorder screening and diagnosis. It is predominantly found in middle-aged females. Though rare, there is a possibility of carcinomas arising within mammary hamartomas, and identification of suspicious features on imaging should prompt evaluation to exclude an underlying tumor. Complete surgical excision is the treatment of choice, though in recent years, lesser invasive ultrasound (US)-guided vacuum-assisted breast biopsy (VABB) has shown encouraging results.
Funding. The Authors declare that they did not receive any grant/funds for this research from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest. The Authors declare that they do not have any relevant financial or non-financial conflicts of interest to report.
Author contributions. Both the authors committed to assume responsibility for the project and contributed in various degrees to data analysis, article drafting, and critical revision.
Data availability statement. Requests for the data used to support the findings may be sent by email to the corresponding author, who would be glad to provide it to anyone.
Patient consent. Patient consent is not applicable because no firsthand cases have been presented and the images are obtained from case reports that have already been published by other renowned academics.
Ethics Committee approval. According to the institution’s policy, literature reviews articles do not need approval from the ethical committee.
Acknowledgement. The publishers who provide the literature in open access and permit reuse for non-commercial uses have been acknowledged by the authors.
References
1. Aminpoura N, Sogunrob O, Towfighia P, Parkc B, Boisvertb M. Clinical management of myoid hamartomas of the breast: a case report and literature review. Heliyon 2022; 8(11): e11723. DOI: 10.1016/j.heliyon.2022.e11723.
2. Türkyilmaz Z, Aydin T, Yilmaz R, Önder S, Özkurt E, Tükenmez M, Müslümanoğlu M, Acunaş G, İğci A, Özmen V, Dinçağ A, Cabioğlu N. Our 20-year institutional experience with surgical approach for breast hamartomas. Eur J Breast Health 2019; 15: 171–175. DOI: 10.5152/ejbh.2019.4624.
3. Crothers JG, Butler NF, Fortt RW, Gravelle IH. Fibroadenolipoma of the breast. Br J Radiol 1985; 58(687): 191–202. DOI: 10.1259/0007-1285-58-687-191.
4. Kopans DB, Meyer JE, Proppe KH. Ultrasonographic, xeromammographic and histologic correlation of a fibroadenolipoma of the breast. J Clin Ultrasound 1982; 10(8): 409–411. DOI: 10.1002/jcu.1870100814.
5. Karki S, Shrestha A, Shrestha B. Adenolipoma of the breast: a case report. JNMA J Nepal Med Assoc 2021; 59(243): 1189–1191. DOIi: 10.31729/jnma.6925.
6. Tazeoğlu D, Dağ A, Arslan B, Berkeşoğlu M. Breast hamartoma: clinical, radiological, and histopathological evaluation. Eur J Breast Health 2021; 17(4): 328–332. DOI: 10.4274/ejbh.galenos.2021.2021-3-6.
7. Wahner-Roedler DL, Sebo T, Gisvold J. Hamartomas of the breast: clinical, radiologic, and pathologic manifestations. Breast J 2001; 7: 101–105. DOI: 10.1046/j.1524-4741.2001.007002101.x.
8. Farrokh D, Hashemi J, Ansaripour E. Breast hamartoma: mammographic findings. Iran J Radiol 2011; 8(4): 258–260. DOI: 10.5812/iranjradiol.4492.9.
Sevim Y, Kocaay AF, Eker T, Celasin H, Karabork A, Erden E, Genc V. Breast hamartoma: a clinicopathologic analysis of 27 cases and a literature review. Clinics (Sao Paulo) 2014; 69(8): 515–523. DOI: 10.6061/clinics/2014(08)03.
10. Ilkay Eren Karanis M, Kucukkosmanoglu I, Altunkeser A, Koksal H. Clinicopathological analysis of seven breast hamartomas and review of the literature. Annals of Medical Research 2021; 27(1): 271–276. DOI: 10.5455/annalsmedres.2019.09.537.
11. Phan VT, Nguyen NT, He J, Robinson AS, Nguyen QD. A male patient with breast hamartoma: an uncommon finding. Cureus 2020; 12(7): e9444. DOI: 10.7759/cureus.9444.12.
Amir RA, Sheikh SS. Breast hamartoma: a report of 14 cases of an under-recognized and under-reported entity. Int J Surg Case Rep 2016; 22: 1–4. DOI: 10.1016/j.ijscr.2016.03.007.
13. Lee WF, Sheen-Chen SM, Chi SY, Huang HY, Ko SF. Hamartoma of the breast: an underrecognized disease? Tumori 2008; 94(1): 114–115. DOI: 10.1177/030089160809400120.
14. Ruiz Tovar J, Reguero Callejas ME, Aláez Chillarón AB, Ramiro Pérez C, Collado Guirao MV, Rojo Blanco R, Muñoz Martín-Cámara J, González-Palacios F, García Villanueva A. Mammary hamartoma. Clin Transl Oncol 2006; 8(4): 290–293. DOI: 10.1007/BF02664941.15.
15. Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist 2006; 11(5): 435–449. DOI: 10.1634/theoncologist.11-5-435.
16. Herbert M, Sandbank J, Liokumovich P, Yanai O, Pappo I, Karni T, Segal M. Breast hamartomas: clinicopathological and immunohistochemical studies of 24 cases. Histopathology 2002; 41(1): 30–34. DOI: 10.1046/j.1365-2559.2002.01429.x.
17. Kajo K, Zubor P, Danko J. Myoid (Muscular) hamartoma of the breast: case report and review of the literature. Breast Care (Basel) 2010; 5(5): 331–334. DOI: 10.1159/000321341.
18. Tse GM, Law BK, Ma TK, Chan AB, Pang LM, Chu WC, Cheung HS. Hamartoma of the breast: a clinicopathological review. J Clin Pathol 2002; 55(12): 951–954. DOI: 10.1136/jcp.55.12.951.
19. Alran L, Chamming’s F, Auriol-Leizagoyen S, Velasco V, Deleau F, Brouste V, Bonhomme B, Ben Rejeb H, Marty M, MacGrogan G. Breast hamartoma: reassessment of an under-recognised breast lesion. Histopathology 2022; 80(2): 304–313. DOI: 10.1111/his.14544.
20. Chiacchio R, Panico L, D’Antonio A, Delrio P, Bifano D, Avallone M, Pettinato G. Mammary hamartomas: an immunohistochemical study of ten cases. Pathol Res Pract 1999; 195(4): 231–236.
21. Presazzi A, Di Giulio G, Calliada F. Breast hamartoma: ultrasound, elastosonographic, and mammographic features. Mini pictorial essay. J Ultrasound 2015; 18(4): 373–377. DOI: 10.1007/s40477-015-0175-0.
22. Ahire PP, Gandhi AS, Jaiswal Y, Binorkar A, Joshi PN, Sukumaran G. Mastering the massive: the surgical strategy and outcomes in a case of a large breast hamartoma. Cureus 2024; 16(11): e73896. DOI: 10.7759/cureus.73896.
23. Rumpf A, Mathiak M, Schäfer F, Caliebe A, Farrokh A, Elessawy M, Bauerschlag D, Maass N, van Mackelenbergh M, Heilmann T. A giant mammary hamartoma in a young breast cancer patient. Breast Care 2021; 16(1): 85–88. DOI: 10.1159/000507604.
24. Palo S, Agrawal V. Giant myoid mammary hamartoma: a case report. J Cancer Res Ther 2024; 20(3): 1103–1105. DOI: 10.4103/jcrt.jcrt_2154_22.
25. Mohamed A. Breast hamartoma: unusual radiological presentation. Radiol Case Rep 2020; 15(12): 2714–2717. DOI: 10.1016/j.radcr.2020.10.015.
26. El Yousfi Z, El Mansoury FZ, El Bakkari A, Omor Y, Latib R. Breast hamartoma with synchronous contralateral breast cancer: a case report. Cureus 2024; 16(8): e66534. DOI: 10.7759/cureus.66534.27.
Cucci E, Santoro A, Di Gesú C, Ciuffreda M, Maselli G, Pierro A, Sallustio G. Integrated imaging of breast hamartoma: two case reports. Breast Dis 2015; 35(1): 53–57. DOI: 10.3233/BD-140379.
28. Erdem G, Karakaş HM, Işık B, Fırat AK. Advanced MRI findings in patients with breast hamartomas. Diagn Interv Radiol 2011; 17(1): 33–37.
29. Vasei N, Shishegar A, Ghalkhani F, Darvishi M. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis 2019; 18(1): 139. DOI: 10.1186/s12944-019-1078-4.
30. Krishnamoorthy R, Savasere T, Prabhuswamy VK, Babu R, Shivaswamy S. Giant malignant phyllodes tumour of breast. Case Rep Oncol Med 2014; 2014: 956856. DOI: 10.1155/2014/956856.
31. Lee EH, Wylie EJ, Bourke AG, Bastiaan De Boer W. Invasive ductal carcinoma arising in a breast hamartoma: two case reports and a review of the literature. Clin Radiol 2003; 58(1): 80–83. DOI: 10.1053/crad.2003.1133.
32. Choi N, Ko ES. Invasive ductal carcinoma in a mammary hamartoma: case report and review of the literature. Korean J Radiol 2010; 11(6): 687–691. DOI: 10.3348/kjr.2010.11.6.687.
33. Soltani K, Taghdiri M, Keshavarz E, Mohammadi S. A rare case of breast hamartoma containing a lesion with malignant presentation on radiologic evaluation and benign presentation on pathologic findings: a case report. Radiol Case Rep 2024; 20(1): 239–242. DOI: 10.1016/j.radcr.2024.10.037.
34. Kuroda N, Sugimoto T, Numoto S, Enzan H. Microinvasive lobular carcinoma associated with intraductal spread arising in a mammary hamartoma. J Clin Pathol 2002; 55(1): 76–77. DOI: 10.1136/jcp.55.1.76.
35. Ruiz-Tovar J, Reguero-Callejas ME, Aláez AB, Ramiro C, Rojo R, Collado MV, González-Palacios F, Muñoz J, García-Villanueva A. Infiltrating ductal carcinoma and ductal carcinoma in situ associated with mammary hamartoma. Breast J 2006; 12(4): 368–370. DOI: 10.1111/j.1075-122X.2006.00279.x.
36. Li M, Lin G, You W, Zhen W, Xu C, Hong J, Ye D, Dong S. Hamartoma of the breast in a man: a rare case report. Medicine (Baltimore) 2019; 98(50): e18372. DOI: 10.1097/MD.0000000000018372.
37. Chang HL, Lerwill MF, Goldstein AM. Breast hamartomas in adolescent females. Breast J 2009; 15(5): 515–520. DOI: 10.1111/j.1524-4741.2009.00769.x.
38. Gupta SS, Singh O, Hastir A, Arora G, Sabharwal G, Mishra H. Breast hamartoma with intrathoracic extension in a 13-year-old boy. J Cancer Res Ther 2010; 6(1): 86–88. DOI: 10.4103/0973-1482.63559.
39. Cazorla S, Arentz C. Breast hamartomas – differential consideration in slow developing breast asymmetry. JPRAS (Open) 2015; 3: 17–21. DOI: 10.1016/j.jpra.2014.12.004.
40. Hu H, Zhang M, Liu Y, Li X, Liu G, Wang Z. Mammary hamartoma: is ultrasound-guided vacuum-assisted breast biopsy sufficient for its treatment? Gland Surgery 2020; 9(5): 1278–1285. DOI: 10.21037/gs-20-437.
41. Hanby A, Walker C, Tavassoli FA, Devilee P. Pathology and genetics: tumours of the breast and female genital organs. WHO classification of tumours series – volume IV. Lyon, France: IARC Press. Breast Cancer Research 2004; 6: 103.
42. Hanson CA, Snover DC, Dehner LP. Fibroadenomatosis (fibroadenomatoid mastopathy): a benign breast lesion with composite pathologic features. Pathology 1987; 19(4): 393–396. DOI: 10.3109/00313028709103889.