Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2025, vol. 24(3), pp. 212–220 DOI: https://doi.org/10.15388/LietChirur.2025.24(3).5
Concurrent Occurrence of Rectal Adenocarcinoma and Vulvar Keratinizing Squamous Cell Carcinoma: Case Report and Literature Review
Odeta Laukaitytė
Lietuvos sveikatos mokslų universitetas, Medicinos fakultetas, Kaunas, Lietuva
Lithuanian University of Health Sciences, Faculty of Medicine, Kaunas, Lithuania
El. paštas laukaityte.odeta@gmail.com
https://ror.org/0069bkg23
Gustė Brazytė
Lietuvos sveikatos mokslų universitetas, Medicinos fakultetas, Kaunas, Lietuva
Lithuanian University of Health Sciences, Faculty of Medicine, Kaunas, Lithuania
El. paštas gustebraz@gmail.com
https://ror.org/0069bkg23
Tadas Latkauskas
Lietuvos sveikatos mokslų universiteto ligoninė Kauno klinikos, Chirurgijos klinika, Kaunas, Lietuva
Hospital of Lithuanian University of Health Sciences Kauno Klinikos, Department of Surgery, Kaunas, Lithuania
El. paštas tadas.latkauskas@kaunoklinikos.lt
https://ror.org/0069bkg23
Saulius Paškauskas
Lietuvos sveikatos mokslų universiteto ligoninė Kauno klinikos, Akušerijos ir ginekologijos klinika, Kaunas, Lietuva
Hospital of Lithuanian University of Health Sciences Kauno Klinikos, Department of Obstetrics and Gynecology, Kaunas, Lithuania
El. paštas saulius.paskauskas@kaunoklinikos.lt
https://ror.org/0069bkg23
Abstract. Introduction. Vulvar carcinoma is a rare malignancy, whereas rectal cancer is a more common oncological condition. However, the simultaneous occurrence of these two diseases is exceptionally rare. Case presentation. A 76-year-old woman presented with rectal bleeding and was previously diagnosed with keratinizing squamous cell carcinoma G1 of the vulva, unrelated to HPV. Imaging revealed tumor-like masses in the vulva and rectum, with pathological lymph nodes. Biopsy confirmed rectal adenocarcinoma in situ. She underwent obstructive rectal resection, colostomy formation, vulvar resection, and lymphadenectomy. Postoperative antibiotic therapy was administered and discontinued after seven days due to stable inflammatory markers. The patient was discharged in good condition, with planned rehabilitation in a sanatorium. Conclusion. Rectal adenocarcinoma and vulvar keratinizing squamous cell carcinoma concurrent occurrence is very rare. The risk factors for vulvar cancer (HPV, smoking, immunosuppression, lichen sclerosus) and rectal cancer (genetic predispositions, lifestyle factors (e.g., smoking, alcohol consumption), inflammatory bowel diseases) differ. However, genetic syndromes and certain interactions between lifestyle factors may lead to the concurrent occurrence of these two tumors. Given the complexity of managing two primary tumors, a multidisciplinary surgical approach was essential in our case. Further research is needed to explore possible biological links and optimize diagnostic and treatment strategies for such rare oncological presentations.
Keywords: rectal carcinoma, vulvar carcinoma, vulvar cancer risk factors, rectal cancer risk factors, vulvar squamous cell carcinoma and rectal adenocarcinoma.
Tiesiosios žarnos adenokarcinomos ir vulvos ragėjančios plokščialąstelinės karcinomos pasireiškimas kartu: klinikinio atvejo analizė ir literatūros apžvalga
Santrauka. Įvadas. Vulvos karcinoma – retas piktybinis navikas. Tiesiosios žarnos vėžys – dažnesnis onkologinis susirgimas. Abi šios ligos kartu pasireiškia itin retai. Klinikinis atvejis. 76 m. moteris kreipėsi dėl kraujavimo iš tiesiosios žarnos. Prieš tai pacientei ambulatoriškai diagnozuota vulvos ragėjanti plokščialąstelinė karcinoma (G1), nesusijusi su žmogaus papilomos virusu (ŽPV). Vaizdiniai tyrimai atskleidė esant navikines mases vulvoje ir tiesiojoje žarnoje bei patologinius limfmazgius. Atlikus biopsiją, patvirtinta tiesiosios žarnos naviko adenokarcinoma in situ. Pacientei atlikta obstrukcinė tiesiosios žarnos rezekcija, suformuojant kolostomą, vulvos rezekcija ir limfadenektomija. Pooperaciniu laikotarpiu taikyta antibiotikoterapija. Ji nutraukta po septynių dienų dėl stabilių uždegiminių rodiklių. Pacientė išleista iš ligoninės geros būklės, jai suplanuota reabilitacija sanatorijoje ir adjuvantinė terapija. Išvada. Tiesiosios žarnos adenokarcinoma ir vulvos ragėjanti plokščialąstelinė karcinoma kartu pasireiškia itin retai. Vulvos vėžio rizikos veiksniai (ŽPV, rūkymas, imunosupresija, lichen sclerosus) ir tiesiosios žarnos vėžio rizikos veiksniai (genetinė predispozicija, gyvenimo būdo veiksniai, tokie kaip rūkymas, alkoholio vartojimas, uždegiminės žarnyno ligos) skiriasi. Vis dėlto tam tikri genetiniai sindromai ir gyvenimo būdo veiksnių sąveikos gali lemti šių dviejų atskirų navikų išsivystymą. Kalbamuoju atveju, atsižvelgus į dviejų pirminių navikų gydymo sudėtingumą, galima teigti, kad itin svarbus multidisciplininis chirurginis gydymas. Būtini tolesni tyrimai, siekiant nustatyti galimus biologinius ryšius ir optimizuoti tokių retų onkologinių atvejų diagnostikos ir gydymo strategijas.
Reikšminiai žodžiai: tiesiosios žarnos karcinoma, vulvos karcinoma, vulvos vėžio rizikos veiksniai, tiesiosios žarnos vėžio rizikos veiksniai, vulvos plokščialąstelinė karcinoma, tiesiosios žarnos adenokarcinoma.
Received: 2025-03-28. Accepted: 2025-04-26.
Copyright © 2025 Odeta Laukaitytė, Gustė Brazytė, Tadas Latkauskas, Saulius Paškauskas. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
A significant proportion of malignant tumors originate from the skin of the labia, while those arising from the clitoris or vestibular glands are exceedingly rare [1]. Vulvar cancer accounts for only 4% of all gynecological malignancies, and as of 2020, it represented 0.8% of all cancers globally [2, 3]. The most common subtype of vulvar cancer is squamous cell carcinoma, comprising more than 90% of all vulvar carcinomas. Globally, the incidence of vulvar cancer reaches approximately 30 000 cases annually [2]. Vulvar cancer predominantly affects postmenopausal women [1].
The incidence of colorectal cancer continues to rise annually, particularly in developing countries. It is the third most common malignancy and the second leading cause of cancer-related mortality worldwide [4]. In 2018, the number of colorectal cancer cases reached 1.8 million [5, 6]. Rectal cancer accounts for approximately one-third of all colorectal cancer cases [6]. About 90% of colorectal carcinomas are adenocarcinomas [5]. According to the literature, approximately 65% of colorectal cancer cases occur sporadically, without a family history or inherited genetic mutations [5]. The remaining cases are associated with familial factors, with 5% of these being attributed to hereditary cancer syndromes [5].
The concurrent occurrence of rectal adenocarcinoma and keratinizing squamous cell carcinoma of the vulva is exceedingly rare and is sparsely documented in the literature. Thus, we aim to present this unique case observed in our clinic. Additionally, we analyzed clinical cases similar to ours (Table 1) and conducted a literature review to assess the risk factors for vulvar and rectal cancers.
Case presentation
A 76-year-old woman with type II diabetes mellitus and arterial hypertension presented with complaints of rectal bleeding during defecation. Earlier, she had reported itching and lesions in the vulvar region, leading to outpatient evaluation for vulvar carcinoma. A biopsy confirmed keratinizing squamous cell carcinoma G1, unrelated to HPV. Cancer marker tests were performed: CA19-9 level was 38.6 U/mL, and CEA level was 46.6 ng/mL. Contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis revealed tumor-like masses involving the labia minora and vagina, as well as suspicious changes in the rectum. Pathological lymph nodes were identified in the inguinal region, measuring up to 3.4x1.7 cm, and pararectally, up to 1 cm in size. Fibrocolonoscopy revealed a circumferential, ulcerated, lumen-narrowing tumor extending from 5 cm to 15 cm above the anal verge. Biopsy from the rectal tumor confirmed adenocarcinoma in situ, unclassified. For further assessment, magnetic resonance imaging (MRI) with intravenous contrast was performed (Figure 1). A tumor measuring approximately 7.9 cm in length was detected in the middle third of the rectum, growing into the intestinal lumen and invading all layers of the rectal wall in certain areas. The tumor exhibited DWI restriction features. Several lymph nodes in the mesorectum appeared pathological. According to MRI imaging findings, the rectal cancer is classified as cT3N1, MRF (‒).
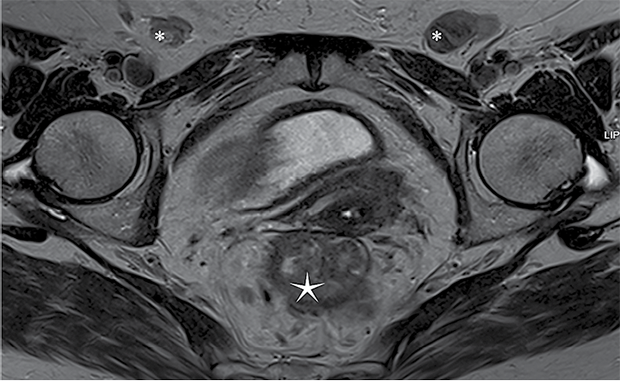
Figure 1. MRI
* ‒ clusters of enlarged lymph nodes, ‒ cancerous mass in rectal region.
The patient underwent surgery via a laparotomy incision, which included obstructive rectal resection with the formation of a colostomy on the left side of the abdomen. A pathological lymph node near the left external iliac artery (a. iliaca externa sin.) was excised. A drainage tube was inserted into the pelvis through the left iliac region. Through separate skin incisions in both groins, enlarged clusters of lymph nodes, fixed to subcutaneous tissues and resembling pathological nodes, were removed. Bilateral vulvectomy was performed, including removal of the clitoris, with 1 cm margins of healthy tissue and resection of approximately 5 mm of the posterior vaginal wall at the vaginal entrance (Figure 2). Antibiotic therapy with cefuroxime and metronidazole was initiated due to the high risk of infection associated with an open bowel lumen during surgery, and preoperative antibiotic prophylaxis with cefazolin was administered.
Histological examination of postoperative specimen confirmed infiltrative colonic adenocarcinoma, classified as pT3, N1b, Mx, LVi0, R0, G2, with metastases found in 6 out of 18 examined lymph nodes within the surrounding adipose tissue of the colon. Histological examination of another specimen revealed keratinizing squamous cell carcinoma of the vulva, unrelated to HPV, classified as pT1b, N2c, R1, G1, with metastases detected in one para-iliac lymph node, three lymph nodes in the left groin, and two lymph nodes in the right groin.
Antibiotic therapy was discontinued after seven days as inflammatory markers did not progress. Observing a favorable clinical condition, the patient was discharged home 12 days postoperatively (Figure 3). A third-stage rehabilitation program and radiotherapy were planned.
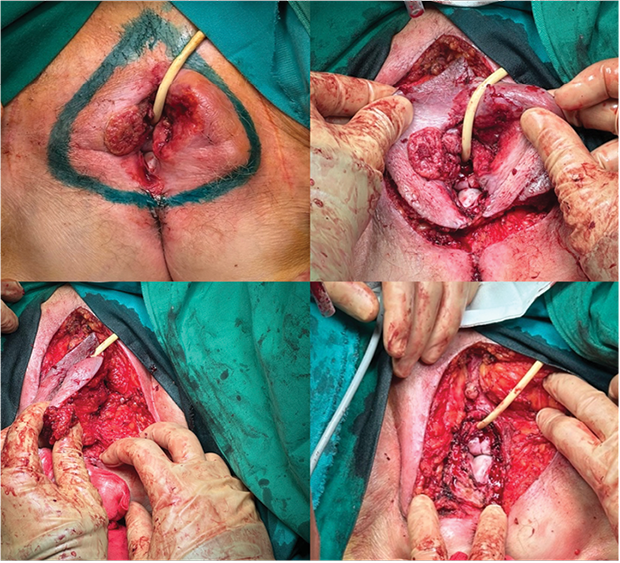
Figure 2. Vulvar resection
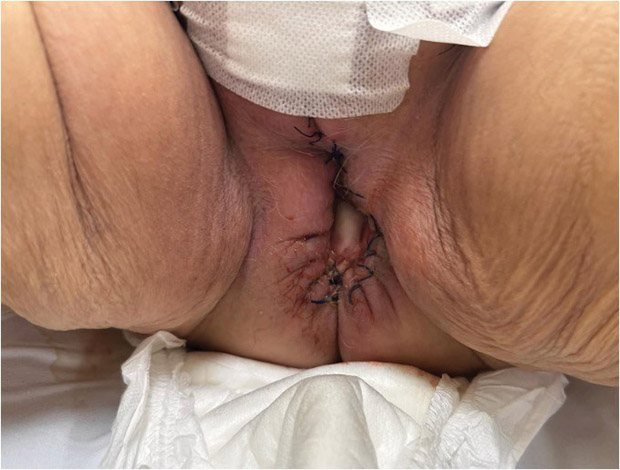
Figure 3. The appearance of the vulva on the day of discharge from the hospital
Discussion
Vulvar squamous cell carcinoma (VSCC) develops through two distinct pathways: an HPV-associated form, primarily affecting younger women, especially those who smoke or are immunosuppressed [1, 2], and an HPV-independent form, most common in postmenopausal women with lichen sclerosus (LS) [1, 2]. High-risk HPV types 16 and 18 are linked to 69% of VSCC cases [7], with HSIL as a precancerous lesion [1, 2], whereas LS is strongly associated with differentiated vulvar intraepithelial neoplasia (dVIN), which progresses more aggressively to carcinoma [8‒10].
Colorectal cancer arises through genetic and epigenetic changes in colonic epithelium, driven by environmental and lifestyle factors [5]. Risk increases with age, male gender [4, 5], poor diet, alcohol consumption [5, 11‒14], smoking [15], and genetic syndromes such as Lynch syndrome and familial adenomatous polyposis [16, 17]. Long-term dietary patterns, particularly low fiber and high red meat intake have been associated with an elevated colorectal cancer risk [5].
Given the distinct etiology and progression of these malignancies, treatment strategies must be tailored accordingly. Below, we discuss the management approaches for vulvar and rectal cancer.
Managing vulvar cancer typically includes a combination of surgical intervention and radiation therapy, in early stage cancer sentinel lymph node biopsy (SNB) procedure is performed. Surgical treatment for vulvar cancer depends on tumor size, location, and lymph node involvement [18]. Early-stage tumors (T1a) with ≤1 mm of stromal invasion are managed with wide local excision, while larger tumors (T1b, >1 mm stromal invasion or T2 ≤ 4 cm and the lesion is 1 cm from median line) may require wide local excision or a modified radical vulvectomy with SNB [19]. For advanced cases (T2 > 4 cm, T3 and lesion ≥1 cm from the median line) a radical vulvectomy combined with ipsilateral inguino-femoral lymph node dissection is advised [19].
Postoperative radiotherapy aims to lower recurrence rates and improve survival [18]. Adjuvant therapy is typically reserved for patients with advanced disease, large tumors, or positive lymph nodes [19]. If invasive disease reaches the pathological excision margins of the primary tumor and further surgical excision is not feasible, postoperative radiotherapy is recommended [18]. Radiation is highly effective in preventing recurrence in inguino-femoral lymph nodes, particularly for patients with multiple positive nodes or extracapsular spread [19]. However, its role in cases with a single positive node remains unclear [19].
Rectal cancer treatment typically follows a multimodal strategy [20]. For early-stage (cT1, cN0, M0) rectal cancer local excision is recommended (TAE, TAMIS, TEMS) or sometimes total mesorectal excision (TME) [21]. TME is often preferred for its comprehensive removal of the rectum and associated lymph nodes [21]. For locally advanced disease, treatment includes neoadjuvant chemotherapy, tailored to the clinical situation, followed by total mesorectal excision and subsequent adjuvant chemotherapy [20‒22]. The choice of procedure depends on factors such as tumor size, location, and patient health [21]. In patients achieving a complete clinical response, organ-preserving options like the “watch-and-wait” approach may be pursued to avoid surgery while maintaining comparable outcomes. Total neoadjuvant therapy is an emerging protocol that improves patient compliance and targets micrometastases before surgical intervention. Advanced imaging technologies are crucial for accurate staging, treatment planning, and assessing response to therapy [20, 22].
Six clinical cases were also analyzed [23‒27] (Table 1). The analysis revealed that the concurrent occurrence of rectal adenocarcinoma and keratinizing squamous cell carcinoma of the vulva is extremely rare, with only one documented case involving two distinct tumors [23]. Another similar case involved adenocarcinoma in the cecum [24]. In two other described cases, metastases were observed, but not two separate primary tumors [25, 26]. Additionally, one case associated with a MUTYH gene mutation reported the occurrence of five independent cancers, including rectal and vulvar cancers [27].
The management of such rare synchronous malignancies varies greatly depending on individual tumor characteristics and disease extent. Our clinical case is particulaly rare as it required the integration of distinct treatment strategies for two different malignancies. The therapeutic approaches for vulvar cancer differ significantly from those for rectal cancer, as previously discussed. Typically, in cases like ours, rectal cancer treatment involves neoadjuvant therapy followed by surgery, whereas vulvar cancer is primarily managed with surgery, followed by adjuvant therapy. In our case the decision was made to perform an obstructive rectal resection with colostomy formation, alongside a bilateral vulvectomy and lymphadenectomy. This approach aimed to achieve complete tumor resection while minimizing the risks associated with multiple separate surgical procedures. Given the high risk of infection due to an open bowel lumen, perioperative antibiotic prophylaxis and postoperative antibiotic therapy were administered. The patient’s postoperative course was uneventful, and a multidisciplinary team planned further rehabilitation and adjuvant radiotherapy to optimize long-term oncological outcomes.
Conclussions
The concurrent occurrence of rectal adenocarcinoma and vulvar keratinizing squamous cell carcinoma is exceptionally rare. In this report we reviewed vulvar and rectal cancer risk factors, treatment options and similar clinical cases described in the scientific literature, while also presenting a clinical case observed in our hospital. The treatment strategies for both malignancies depends on tumor size, location, and lymph node involvement.
Our case required a complex treatment str ategy, as it involved the simultaneous management of two distinct oncological processes, posing a significant challenge in selecting the appropriate treatment strategy. During a multidisciplinary team discussion involving gynecologists, surgeons, and oncologists, it was determined that surgical treatment should be prioritized, followed by adjuvant therapy. Based on the histological findings – infiltrative colonic adenocarcinoma (pT3, N1b, Mx, LVi0, R0, G2) and keratinizing squamous cell carcinoma of the vulva, unrelated to HPV (pT1b, N2c, R1, G1) – the chosen treatment strategy was well-adapted. Importantly, no cancer cells were detected at the resection margins of the rectum, confirming clear surgical margins.
Table 1. Analysis of clinical cases of concurrent vulvar and rectal cancer
|
Authors, country, |
Case description |
Findings |
Treatment |
|
Kremzer T, Pete I, Stári O. (Hungary) The primary surgical therapy of a synchronous sigmoid, rectal and vulvar cancer. [23] |
64-year-old woman with vulvar squamous cell carcinoma and rectal/sigmoid adenocarcinoma. |
Histology: sigmoid tumor (pT3N0M0), rectal tumor (pT2N0M0), vulvar tumor (pT1N0M0). |
The patient underwent sigmoid colectomy and rectal extirpation, along with vulvectomy and inguinal lymphadenectomy, followed by colostomy formation. |
|
Vahidfar M, Abdolahi AH, Zarchi M. (Iran) Colorectal and vulvar synchronous cancer. [24] |
63-year-old woman with vulvar squamous cell carcinoma and cecal adenocarcinoma. |
CT: 5 cm hypoechoic liver lesion (metastasis). |
The patient underwent surgical resection of the vulvar lesion and cecal adenocarcinoma, with adjuvant chemotherapy due to suspected hepatic metastasis. |
|
Akpak YK, Dandin Ö, Gün İ, Atay V. (Turkey) A rare case of vulvar skin metastasis of rectal cancer. [25] |
47-year-old woman with a vulvar lesion three years after abdominoperineal resection and oophorectomy for ovarian cancer metastases. |
MRI: hyperintense vulvar lesions; surgical excision revealed atypical glandular structures infiltrating vulvar skin. Referred for oncology and radiotherapy. |
Surgical excision of the vulvar lesion was performed, and the patient was referred for systemic oncological treatment, including chemotherapy and radiotherapy. |
|
Soh JM, Scott GA, Pavlovitz BT. (USA) Metastatic rectal adenocarcinoma and anal squamous cell carcinoma. [26] |
47-year-old woman with primary anal squamous cell carcinoma and vulvar metastases. |
PET/CT: metastases in inguinal/paraaortic lymph nodes; chemotherapy initiated. |
Systemic chemotherapy targeting metastatic disease. |
|
Soh JM, Scott GA, Pavlovitz BT. (USA) Metastatic rectal adenocarcinoma and anal squamous cell carcinoma. [26] |
49-year-old woman with stage IV rectal adenocarcinoma and vulvar metastases. |
PET/CT: metastases in liver, lungs, lymph nodes; treated with radiotherapy and chemotherapy. |
Surgical resection, followed by chemoradiotherapy. Postoperatively, vulvar nodules were identified as metastatic adenocarcinoma, leading to palliative radiotherapy and chemoradiotherapy for metastatic disease. |
|
Arroyave A, Nodit L, Clegg D, Russ A. (USA) Forty-eight-year-old female MUTYH carrier presenting with five concurrent primary cancers. [27] |
48-year-old woman with multiple primary cancers, including rectal adenocarcinoma and vulvar squamous cell carcinoma. |
Genetic testing: MUTYH mutation; underwent extensive surgical and chemoradiotherapy. |
Radical partial vulvectomy, thyroidectomy, neoadjuvant chemoradiotherapy, and subsequent pelvic exenteration, hysterectomy, bilateral salpingo-oophorectomy, sigmoid colectomy, radical vaginectomy, and perineal proctectomy. |
Author contributions
Odeta Laukaitytė ‒ conceptualization, methodology, formal analysis, investigation, writing ‒ original draft, writing ‒ review and editing, visualization.
Gustė Brazytė ‒ conceptualization, methodology, formal analysis, investigation, writing ‒ original draft, writing ‒ review and editing.
Tadas Latkauskas ‒ conceptualization, methodology, formal analysis, investigation, writing ‒ review and editing, visualization.
Saulius Paškauskas ‒ conceptualization, methodology, formal analysis, investigation, writing ‒ review and editing, visualization.
References
1. Olawaiye AB, Cuello MA, Rogers LJ. Cancer of the vulva: 2021 update. Int J Gynaecol Obstet 2021; 155: 7–18.
2. Yang H, Almadani N, Thompson EF, Tessier-Cloutier B, Chen J, Ho J, Senz J, McConechy MK, Chow C, Ta M, Cheng A, Karnezis A, Huvila J, McAlpine JN, Gilks B, Jamieson A, Hoang LN. Classification of vulvar squamous cell carcinoma and precursor lesions by p16 and p53 immunohistochemistry: considerations, caveats, and an algorithmic approach. Mod Pathol 2023; 36(6): 100145.
3. Huang J, Chan SC, Fung YC, Pang WS, Mak FY, Lok V, Zhang L, Lin X, Lucero-Prisno 3rd DE, Xu W, Zheng ZJ, Elcarte E, Withers M, Wong MCS, NCD Global Health Research Group, Association of Pacific Rim Universities (APRU). Global incidence, risk factors and trends of vulvar cancer: a country-based analysis of cancer registries. Int J Cancer 2023; 153(10): 1734–1745.
4. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019; 14(2): 89‒103.
5. Pinheiro M, Moreira DN, Ghidini M. Colon and rectal cancer: an emergent public health problem. World J Gastroenterol 2024; 30(7): 644‒651.
6. Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol 2020; 17(7): 414‒429.
7. National Cancer Institute. HPV and Cancer. Cancer.gov, 2019. Available at <https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer>.
8. Weinberg D, Gomez-Martinez RA. Vulvar cancer. Obstet Gynecol Clin North Am 2019; 46(1): 125–135.
9. Leis M, Singh A, Li C, Ahluwalia R, Fleming P, Lynde CW. Risk of vulvar squamous cell carcinoma in lichen sclerosus and lichen planus: a systematic review. J Obstet Gynaecol Can 2022; 44(2): 182‒192.
10. Voss FO, Thuijs NB, Vermeulen RFM, Wilthagen EA, van Beurden M, Bleeker MCG. The vulvar cancer risk in differentiated vulvar intraepithelial neoplasia: a systematic review. Cancers (Basel) 2021; 13(24): 6170.
11. Lu Y, Li D, Wang L, Zhang H, Jiang F, Zhang R, Xu L, Yang N, Dai S, Xu X, Theodoratou E, Li X. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients 2023; 15(3): 730.
12. Kliemann N, Rauber F, Bertazzi Levy R, Viallon V, Vamos EP, Cordova R, Freisling H, Casagrande C, Nicolas G, Aune D, Tsilidis KK, Heath A, Schulze MB, Jannasch F, Srour B, Kaaks R, Rodriquez-Barranco M, Tagliabue G, Agudo A, Panico S, Ardanaz E, Chirlaque MD, Vineis P, Tumino R, Perez-Cornago A, Munk Andersen JL, Tjønneland A, Skeie G, Weiderpass E, Monteiro CA, Gunter MJ, Millett C, Huybrechts I. Food processing and cancer risk in Europe: results from the prospective EPIC cohort study. Lancet Planet Health 2023; 7(3): e219‒e232.
13. Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF‒AICR Continuous Update Project. Ann Oncol 2017; 28(8): 1788‒1802. DOI: 10.1093/annonc/mdx171.
14. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pellucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015; 112(3): 580‒593. DOI: 10.1038/bjc.2014.579.
15. Dimou N, Yarmolinsky J, Bouras E, Tsilidis KK, Martin RM, Lewis SJ, Gram IT, Bakker MF, Brenner H, Figueiredo JC, Fortner RT, Gruber SB, van Guelpen B, Hsu L, Kaaks R, Kweon SS, Lin Y, Lindor NM, Newcomb PA, Sánchez MJ, Severi G, Tindle HA, Tumino R, Weiderpass E, Gunter MJ, Murphy N. Causal effects of lifetime smoking on breast and colorectal cancer risk: Mendelian randomization study. Cancer Epidemiol Biomarkers Prev 2021; 30(5): 953‒964.
16. Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg 2018; 31(3): 168‒178.
17. Brandaleone L, Dal Buono A, Gabbiadini R, Marcozzi G, Polverini D, Carvello M, Spinelli A, Hassan C, Repici A, Bezzio C, Armuzzi A. Hereditary colorectal cancer syndromes and inflammatory bowel diseases: risk management and surveillance strategies. Cancers (Basel) 2024; 16(17): 2967.
18. Oonk MHM, Planchamp F, Baldwin P, Bidzinski M, Brännström M, Landoni F, Mahner S, Mahantshetty U, Mirza M, Petersen C, Querleu D, Regauer S, Rob L, Rouzier R, Ulrikh E, van der Velden J, Vergote I, Woelber L, van der Zee AGJ. European Society of Gynaecological Oncology Guidelines for the management of patients with vulvar cancer. Int J Gynecol Cancer 2017; 27(4): 832‒837.
19. Merlo S. Modern treatment of vulvar cancer. Radiol Oncol 2020; 54(4): 371‒376.
20. Sun W, Al-Rajabi R, Perez RO, Abbasi S, Ash R, Habr-Gama A. Controversies in rectal cancer treatment and management. Am Soc Clin Oncol Educ Book 2020; 40: 1‒11.
21. National Institute for Health and Care Excellence. Overview. Colorectal cancer 2020. Available at <https://www.nice.org.uk/guidance/ng151>.
22. Chehade L, Dagher K, Shamseddine A. Tailoring treatment for locally advanced rectal cancer. Cancer Treat Res Commun 2024; 41: 100847.
23. Kremzer T, Pete I, Stári O, Lóderer Z. Szinkron szigmabél-, végbél- és szeméremtesti daganat primer műtéti ellátása. Esetismertetés [The primary surgical therapy of a synchronous sigmoid, rectal and vulvar cancer]. Magy Onkol 2022; 66(2): 153‒156.
24. Vahidfar M, Hossein Abdolahi A, Karimi Zarchi M. Colorectal and vulvar synchronous cancer: a case report. Iranian Journal of Blood and Cancer 2013; 5(4): 151‒152.
25. Akpak YK, Dandin Ö, Gün İ, Atay V, Haholu A. A rare case of vulvar skin metastasis of rectal cancer after surgery. Int J Dermatol 2014; 53(6): e337‒e338.
26. Soh JM, Scott GA, Pavlovitz BT, Mercurio MG. Metastatic rectal adenocarcinoma and anal squamous cell carcinoma masquerading as vulvar lymphangioma circumscriptum. J Obstet Gynaecol 2016; 36(7): 876‒878.
27. Arroyave A, Nodit L, Clegg D, Russ A. Forty-eight-year-old female MUTYH carrier presenting with five concurrent primary cancers. Cancer Rep (Hoboken) 2022; 5(2): e1455.
28. Bucchi L, Pizzato M, Rosso S, Ferretti S. New insights into the epidemiology of vulvar cancer: systematic literature review for an update of incidence and risk factors. Cancers (Basel) 2022; 14(2): 389.
29. Kraus CN. Vulvar lichen sclerosus. JAMA Dermatol 2022; 158(9): 1088.