Neurologijos seminarai ISSN ISSN 1392-3064 / eISSN 2424-5917
2024, 28(100), pp.126–133 DOI: https://doi.org/10.15388/NS.2024.28.100.6
Klinikinis atvejis / Case Reports
Erika Jokubauskaitė*
Faculty of Medicine, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
Patricija Skučaitė
Faculty of Medicine, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
Ieva Vienažindytė
Department of Neurology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
Neringa Balčiūnienė
Department of Neurosurgery Intensive Care Unit, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
Summary. Pontine and extrapontine myelinolysis represents rare but serious neurological complications associated with the rapid correction of severe hyponatremia. We present a case report of a 49-year-old male who developed pontine and extrapontine myelinolysis following a rapid correction of severe hyponatremia. Initially, the patient was admitted to a district hospital on February 18, 2024 for generalized weakness and an unspecified infection, during which, his sodium level was found to be critically low at 98.6 mmol/L. Rapid treatment raised his sodium to 140 mmol/L over a short period, completing the correction within 10 days. One day after discharge, he was readmitted to the Emergency Department at the Lithuanian University of Health Sciences Hospital Kaunas Clinics on February 29, 2024, with symptoms including altered consciousness, bulbar symptoms, and limb weakness. A head Computed Tomography (CT) scan was performed in the emergency department, which ruled out cerebrovascular accidents and other structural changes in the brain. For further evaluation, the patient was admitted to the Neurology Department, where it was decided to perform a brain Magnetic Resonance Imaging (MRI) scan. As respiratory failure developed, the patient was transferred to the Neurosurgery Intensive Care Unit (NICU). The brain MRI results confirmed pontine and extrapontine myelinolysis. Based on a review of the scientific literature, treatment with methylprednisolone was initiated. The condition was complicated by septic shock with Multiple Organ Dysfunction Syndrome (MODS), with a potential infection source in the lungs. Despite the treatment provided, the patient passed away after five days in NICU. This case highlights that rapid sodium correction can lead to serious complications, such as myelinolysis. It underscores the necessity for gradual sodium correction in patients with severe hyponatremia so that to prevent irreversible neurological damage.
Keywords: Pontine and extrapontine myelinolysis, osmotic demyelination syndrome, hyponatremia, hyponatremia correction, case report.
Santrauka. Tilto ir ekstrapontinė mielinolizė yra reta neurologinė staigios hiponatremijos korekcijos komplikacija. Šis klinikinis atvejis yra 49 metų vyro, kuriam išsivystė tilto ir ekstrapontinė mielinolizė po greitos bei komplikuotos hiponatremijos korekcijos. Iš pradžių pacientas buvo hospitalizuotas vienoje regiono ligoninėje dėl bendro silpnumo ir tiksliai nenustatytos infekcijos, jam tuo metu natrio kiekis kraujo serume buvo ypač mažas – 98,6 mmol/L. Gydymo metu natrio lygis per trumpą laiką pakilo iki 140 mmol/L. Išleistas iš ligoninės, pacientas netrukus, 2024 m. vasario 29 d., nuvežtas į Lietuvos sveikatos mokslų universiteto ligoninės Kauno klinikų Traumų ir skubios pagalbos centrą. Jam buvo sąmonės sutrikimai, bulbariniai simptomai ir galūnių silpnumas. Pacientui Skubios pagalbos skyriuje buvo atlikta galvos smegenų kompiuterinė tomografija. Ji nerodė galvos smegenų kraujotakos sutrikimo ar kitų struktūrinių pakitimų. Nuodugnesniam ištyrimui pacientas buvo paguldytas į Neurologijos skyrių. Ten buvo nuspręsta atlikti galvos smegenų magnetinio rezonanso tyrimą. Išsivysčius ūmiam kvėpavimo funkcijos nepakankamumui, atlikus tyrimą pacientas iš karto buvo perkeltas į Neurochirurgijos intensyviosios terapijos skyrių. Galvos smegenų magnetinio rezonanso tyrimas parodė tilto ir ekstrapontinę mielinolizę. Išanalizavus mokslinę literatūrą, pacientą nuspręsta pradėti gydyti metilprednizolonu. Būklė komplikavosi sepsiniu šoku su dauginiu organų disfunkcijos sindromu (DODS), įtarta infekcija plaučiuose. Nepaisant skirtos antibiotikoterapijos ir kito gydymo, pacientas po 5 dienų mirė. Šis atvejis atskleidžia, kad greita natrio korekcija gali sukelti rimtų komplikacijų, tokių kaip tilto mielinolizė. Tai rodo, kad pacientams, sergantiems sunkia hiponatremija, natrio lygio koregavimas, siekiant išvengti negrįžtamos galvos smegenų pažaidos, turėtų būti laipsniškas.
Raktažodžiai: tilto ir ekstrapontinė mielinolizė, pontinė mielinolizė, osmosinės demielinizacijos sindromas, hiponatremija, hiponatremijos korekcija.
________
* Address: Erika Jokubauskaitė, Faculty of Medicine, Medical Academy, Lithuanian University of Health Sciences, Mickevičiaus 9, LT-44307, Kaunas, Lithuania. Email: erikjoku0124@stud.lsmu.lt
Received: 29/11/2024. Accepted: 21/02/2025
Copyright © Erika Jokubauskaitė, Patricija Skučaitė, Ieva Vienažindytė, Neringa Balčiūnienė, 2024. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Central Pontine Myelinolysis (CPM) is a rare but severe neurological condition characterized by the loss of myelin in the central pons, a crucial part of the brainstem responsible for coordinating the motor and sensory functions. First described by Adams et al. in 1959, CPM is most often associated with the rapid correction of chronic hyponatremia. The sudden shift in sodium levels leads to osmotic stress, resulting in cellular dehydration and disruption of the blood-brain barrier, primarily affecting the myelinated nerve fibers in the pons. The neurological complications resulting from an overly rapid correction are referred to as Osmotic Demyelination Syndrome (ODS). This condition typically arises in patients with underlying conditions such as alcoholism, malnutrition, liver disease, or post-liver transplantation [1]. Clinical features of CPM typically begin to emerge within several days following the rapid correction of hyponatremia. These manifestations can vary widely, ranging from encephalopathy to more severe outcomes such as quadriplegia, pseudobulbar palsy, dysarthria, and dysphagia. In the most critical cases, patients may develop a locked-in syndrome, coma, or even death. While extrapontine regions may also be affected (extrapontine myelinolysis), the classic neurological deficits are primarily associated with damage to the central pons [2]. Diagnostic confirmation of CPM relies on identifying characteristic demyelinating lesions in the pons and/or extrapontine regions via Magnetic Resonance Imaging (MRI) in patients with compatible clinical presentation. Management is primarily supportive, with an emphasis on preventing CPM through the gradual and controlled correction of electrolyte imbalances [3]. This case report discusses the case of a patient with a unique presentation of CPM following a rapid sodium correction after extreme hyponatremia, emphasizing the importance of slow sodium repletion and highlighting the challenges in managing this potentially devastating condition.
A 49-year-old male patient was transported to the Emergency Department (ED) of the Lithuanian University of Health Sciences Hospital Kaunas Clinics from a district hospital on February 29, 2024, due to unspecified altered consciousness, bulbar symptoms, and limb weakness. Two days before the event (on February 27), the patient was discharged from the internal medicine department where he had been treated for an unspecified infection and generalized weakness for 11 days. No other markable symptoms were noted during that time. It was known from medical documentation that the patient had an upper respiratory tract infection, as diagnosed by his family medicine doctor on February 2. Due to generalized weakness, he fell several times, resulting in multiple hematomas observed on the body. Head Computed Tomography (CT) was performed in the internal medicine department, which showed no cerebrovascular disorders or hemorrhage; chest CT showed no signs of pulmonary embolism, but inflammatory changes were noted. Also, severe hyponatremia of 98.6 mmol/L (reference range from district hospital 136–146 mmol/L) was identified based on district hospital tests, which improved to 140 mmol/L in 10 days (Table 1). Hyponatremia was corrected to the lower bound of the normal range within 8 days. The serum potassium level was 3.45 mmol/L, which is slightly below the normal range (3.5–5.4 mmol/L). Leukocytosis and elevated C-Reactive Protein (CRP) levels were noted, and the patient was treated with 1.5 grams of Cefuroxime intravenously three times daily.
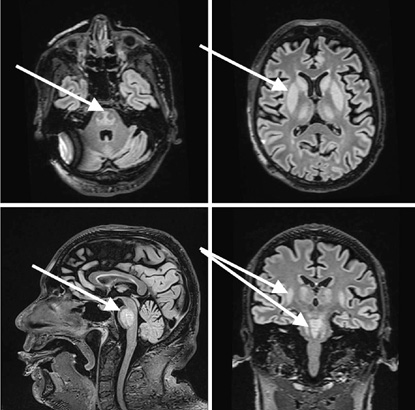
|
Date |
February 18 |
February 19 |
February 20 |
February 22 |
February 26 |
February 28 |
|
Sodium level (mmol/L) |
98.6 |
105 |
110 |
128 |
138 |
140 |
Upon arrival at the ED, the patient’s vital signs were: blood pressure 147/99 mmHg, heart rate 90 beats per minute, respiratory rate 17/min, oxygen saturation 95%, body temperature of 36.2 °C, and Glasgow Coma Scale (GCS) value 10 (the patient spontaneously opened his eyes, was nonverbal, and localized the pain he was experiencing). Pupils: Symmetric and photoreactive. Bulbar symptoms were observed, with excessive salivation and choking. The patient muttered indistinct sounds, did not respond to questions, and failed to follow commands. Notable extensor tone was observed in the extremities, which was more pronounced on the right side than on the left. The pathological Babinski reflex was present on the right. A head CT was performed again, confirming no signs of cerebrovascular issues or hemorrhage. The patient was admitted to the Neurology Department for further investigation, suspecting and trying to differentiate between Posterior Reversible Encephalopathy Syndrome (PRES), vertebrobasilar stroke, or neuroinfection. In the Neurology Department, the patient developed a fever up to 39.2 °C, and a respiratory failure started to progress. Multiple tests were run in the department. A lumbar puncture (glucose 3.87mmol/l, [2.2–3.9], protein 0.50g/l [0.15–0.45], cells 0 x 10^6 /l, white blood cells 0x 10^6 /l) showed no signs of neuroinfection. An electroencephalogram (EEG) was performed, and its results showed no epileptiform changes. A brain MRI was performed under general anesthesia to differentiate between ischemic and hemorrhagic changes, prion disease, PRES syndrome, and inflammatory changes. During the MRI, the patient was intubated; however, due to an ongoing severe respiratory failure requiring mechanical ventilation, the patient was transferred for further treatment to the Neurosurgery Intensive Care Unit. Upon arrival at the Neurosurgery Intensive Care Unit (NICU), the patient had a GCS of 3, with pupils equal and photoreactive. Consciousness was assessed in the context of sedation and muscle relaxants. The patient was on mechanical ventilation, with SpO2 levels between 88–90%. Hemodynamics were stable, with a heart rate of 128 bpm, and a blood pressure of 160/107 mmHg. The capillary refill time was 5 seconds, and his body temperature was 38.7°C. The predominant issues were a severe respiratory failure, an unclear neurological diagnosis, and an uncertain infectious status. Treatment was provided with crystalloids infusion therapy and correction of homeostasis, the patient was sedated with propofol and fentanyl, low molecular weight heparin was given for thromboprophylaxis, thiamine 500 mg three times daily, and magnesium sulfate 25% (to rule out Wernicke’s encephalopathy). Since the CRP and leukocytosis were growing, multiple tests were performed to identify the infectious agent. Blood, bronchial secretion, and urine cultures were taken. Respiratory virus detection tests were taken, as well as antibodies against Treponema pallidum, Human Immunodeficiency Virus (HIV), Herpes Simplex, Borrelia burgdorferi, tick-borne encephalitis, and Epstein-Barr Virus (EBV). MRI Findings showed that a markedly hyperintense area was noted in the mid-dorsal portion of the brainstem on T2-weighted images, with signs of diffusion restriction and no pathological contrast enhancement. Symmetrical, homogeneous T2-weighted hyperintensities were seen in areas around the caudate nucleus, lentiform nucleus, internal capsule (more pronounced dorsally), thalamus, and amygdala, without clear diffusion restriction or pathological enhancement (Figure 1). The MRI findings suggested possible extrapontine and pontine myelinolysis. Liver enzymes and ammonia were within normal limits, as were thyroid hormones and vitamin B12 and folate levels. Respiratory virus detection tests came back negative, as well as antibodies against Treponema pallidum, HIV, Herpes Simplex, Borrelia burgdorferi, tick-borne encephalitis, and EBV. The patient was treated in the Neurointensive Care Unit for 5 days due to coma and respiratory failure caused by acute demyelinating syndrome (osmotic myelinolysis). Given the clinical progression and observed changes, the prognosis was deemed unfavorable. Following neurological recommendations and current literature, a methylprednisolone pulse therapy was initiated, as there was no specific treatment available. Although, no evidence for autoimmune encephalitis was found. A test for antibodies against neuronal surface antigens came back negative. The condition was complicated by septic shock with multiple organ dysfunction syndrome, with a potential infection source in the lungs (the CRP value was 127 mg/l). Broad-spectrum antibiotics tazocin (4.5g; 4/day) and vancomycin (started with 2g with an automatic syringe pump) were administered as the decision was made to treat it as a septic shock with an unknown infection source. Following the methylprednisolone pulse therapy, leukopenia and neutropenia emerged. Sedation was discontinued, and the patient remained with a GCS score of 3. Throughout treatment, the patient’s condition remained very critical, with a worsening trend. Consciousness remained at GCS 3 under sedation, with continued need for mechanical ventilation and vasopressors, whereas septic shock was progressing. By the end of day five, a diminishing cardiac activity was observed, leading to asystole. Resuscitation efforts were initiated according to adult protocols. In the absence of cardiovascular response to resuscitation, the patient was pronounced dead. The patient’s relatives refused the autopsy.

We have presented a case about a patient with severe Osmotic Demyelination Syndrome (ODS), complicated by pontine and extrapontine myelinolysis, secondary to severe hyponatremia correction. Hyponatremia is defined as a medical condition characterized by abnormally low serum sodium levels, typically recognized when sodium concentrations fall below 135 mmol/L [4]. The patient in the case report had a remarkably low serum sodium level of 98.6 mmol/L, which may be one of the lowest levels ever recorded. According to the literature, a serum sodium concentration of 99 mmol/L was noted in a case study, marking it as the lowest previously documented level [5]. Overcorrection of hyponatremia, as outlined by the European guidelines, is defined as an increase in the serum sodium concentration exceeding 10 mmol/L within the first 24 hours, more than 18 mmol/L within the initial 48 hours, or more than 8 mmol/L per day after the first 24 hours [6]. After rapid correction of hyponatremia, cell water loss and the shift of potassium and sodium back into cells initially increase cation concentrations before organic osmolytes are replenished. Within hours, these changes trigger protein aggregation, DNA fragmentation, and markers of programmed cell death. In regions of the brain affected by demyelination, astrocytes and oligodendrocytes start to die, which may be accompanied by the release of inflammatory cytokines and activation of microglia [7]. Studies in rats have demonstrated that demyelination tends to occur in brain regions where osmolytes are reaccumulated more slowly following a rapid correction of hyponatremia [8]. In the case described, the patient’s sodium level rose from 98.6 mmol/L to 140 mmol/L over approximately 10 days, with a significant correction happening between February 20 and February 26. While this time frame exceeds the classic definition of rapid correction ( >10 mmol/L increase in 24 hours, or >18 mmol/L in 48 hours), the sharp rise from 110 mmol/L to 140 mmol/L in as little as six days might still pose a risk for osmotic demyelination, especially in a vulnerable patient. We cannot comment on the doctors’ decision regarding the sodium level correction, as we were only able to analyze retrospective data from the medical records. The diagnosis of pontine myelinolysis is primarily based on the clinical history, combining known risk factors with a range of neurological symptoms, which can include anything from mild weakness (paraparesis) to complete paralysis (paraplegia), locked-in syndrome, or even fatal outcomes. Although biological tests are nonspecific for pontine myelinolysis, they can help raise suspicion, particularly in cases of rapid shifts in plasma osmolality, such as a fast correction of hyponatremia. That is why diagnosis of myelinolysis is complex, as it requires initially ruling out acute neurological conditions that may present with similar symptoms, such as stroke, neuroinfection, or other sudden-onset neurological disturbances. This step is essential to ensure an accurate diagnosis so that to avoid mistreatment. After ruling out other conditions, the diagnosis is confirmed by using brain MRI [9]. It typically reveals hyperintense areas on T2- and FLAIR-weighted sequences, while appearing hypointense on T1-weighted sequences. These typical changes were seen in the patient in this case report. However, these characteristic lesions often become visible only later in the disease course, whereas early MRI scans may be unremarkable. Therefore, repeat imaging may be necessary as the condition is evolving [10]. Treatment of central pontine and extrapontine myelinolysis remains challenging, as no standardized treatment exists. The approach generally focuses on supportive care and minimizing further neurological damage while managing complications. Case reports have indicated that Intravenous Immunoglobulin (IVIG) therapy could be beneficial in managing Central Pontine Myelinolysis (CPM). For example, a 2018-dated report describes a CPM patient whose neurological symptoms worsened before receiving IVIG. While the exact mechanism remains unclear, it is thought that IVIG might help by modulating immune responses associated with demyelination, potentially slowing the disease progression and lessening its severity [11]. Plasmapheresis is also used as a treatment for CPM, with reported cases varying widely in both the frequency and duration of sessions. Meanwhile, treatment cycles ranged from as few as two sessions to as many as ten, and the interval between treatments has varied: in some cases, daily sessions were utilized, while others opted for treatments every other day or twice a week. In one notable case, plasmapheresis extended over seven weeks, ultimately resulting in complete symptom remission observed at a one-year follow-up [12]. Most reported cases showed symptom improvement, with some even achieving full recovery [12.13]. In the treatment of ODS with steroids, most patients exhibited symptom improvement within months after receiving the intravenous steroid therapy, often supplemented with oral tapering. Hagiwara et al. reported a significant recovery in a patient with both pontine and extrapontine myelinolysis following two courses of intravenous methylprednisolone, which resulted in achieving full recovery in a few months. Conversely, Kallakatta et al. found no beneficial effects in six patients treated with intravenous methylprednisolone, concluding that the outcomes were inconclusive regarding the treatment efficacy [14]. In this case, the decision to treat the patient with steroids was made based on the potential benefits observed in other cases of osmotic demyelination syndrome. Given the patient’s deteriorating condition and the absence of effective alternative treatments, initiation of the methylprednisolone pulse therapy aligned with the current treatment guidelines for managing severe demyelination. The medical team was aware of the immunosuppressive effects of methylprednisolone. However, given the timing of the decision and the existing literature, it was determined that the septic shock could be managed, while the progression of brainstem dysfunction was considered to be of a greater concern at that particular moment. In conclusion, this case underscores the complexities involved in diagnosing and managing the severe osmotic demyelination syndrome. The rapid correction of hyponatremia likely played a critical role in the patient’s neurological deterioration, by highlighting the need for careful monitoring and management of electrolyte imbalances. Although treatments such as steroids, plasmapheresis, and intravenous immunoglobulin have shown promise in some cases, the lack of standardized protocols for ODS complicates clinical decision-making. This case illustrates the necessity of a multidisciplinary approach to ensure a timely diagnosis and the appropriate management, ultimately aiming to mitigate neurological damage and improve patient outcomes. In addition to the cases reported in the literature, our case shares notable similarities with a 74-year-old woman who presented with severe hyponatremia and was treated with saline and potassium chloride. Despite initial improvements in her electrolyte levels, she later developed a hyperintense lesion in the median portion of the pons on MRI, indicative of Osmotic Demyelination Syndrome (ODS), which led to irreversible coma and status epilepticus. Like our case, this underscores the delicate balance required when correcting severe hyponatremia and the associated risk of demyelination. The subsequent neurological deterioration resulting in a fatal outcome highlight the unpredictability and devastating nature of ODS, even with corrective measures [15]. This case further emphasizes the significant risks of rapid sodium correction, particularly in vulnerable patients, thereby reinforcing the need for a cautious approach to treatment so that to minimize the long-term risks of demyelination. As further research emerges, it will be essential to refine treatment strategies and enhance our understanding of the pathophysiology underlying ODS so that to provide better care for affected patients.