Neurologijos seminarai ISSN ISSN 1392-3064 / eISSN 2424-5917
2024, 28(100), pp.118–125 DOI: https://doi.org/10.15388/NS.2024.28.100.5
Klinikinis atvejis / Case Reports
Gabrielė Jankauskaitė*
Lithuanian University of Health Sciences, Medical Academy, Faculty of Medicine, Kaunas
Gintarė Žemgulytė
Lithuanian University of Health Sciences, Medical Academy, Faculty of Medicine, Department of Neurology, Kaunas
Renata Balnytė
Lithuanian University of Health Sciences, Medical Academy, Faculty of Medicine, Department of Neurology, Kaunas
Summary. Guillain-Barré Syndrome (GBS) is an acute, immune-mediated neuropathy of the peripheral nervous system, characterized by rapidly progressive, symmetrical weakness of the limbs and the reduction or absence of tendon reflexes. GBS is a rare neurological disorder diagnosed based on the clinical history, neurological examination, cerebrospinal fluid analysis and electroneuromyography (ENMG). In rare cases, GBS can occur together with other syndromes. Posterior Reversible Encephalopathy Syndrome (PRES) is a clinical-radiological condition defined by acute neurological symptoms and characteristic imaging findings. The manifestation of these syndromes may be influenced by congenital Klippel-Feil Syndrome (KFS), which is diagnosed by imaging studies observing fusion of at least two vertebrae of the cervical spine. This syndrome can lead to spinal canal stenosis, reduced neck mobility, and a lower hairline. In this article, we present a 72-year-old female patient who presented to the Emergency Department due to impaired speech and disorientation. Neurological examination revealed findings consistent with encephalopathy. Detailed diagnostic evaluation confirmed a diagnosis of GBS presenting with PRES, accompanied by KFS. This case illustrates the diversity of clinical symptoms in GBS and highlights the importance of a comprehensive differential diagnosis when evaluating patients with suspected demyelinating polyneuropathy.
Keywords: Guillain-Barré syndrome, posterior reversible encephalopathy syndrome, Klippel-Feil syndrome, demyelinating polyneuropathy.
Santrauka. Gijeno-Barė (Guillain-Barré) sindromas (GBS) – ūmi, su imuniniu atsaku susijusi periferinės nervų sistemos liga, kuriai būdingas greitai progresuojantis simetrinis galūnių silpnumas ir sausgyslių refleksų susilpnėjimas ar išnykimas. GBS yra retas neurologinis sutrikimas, kurio diagnostika pagrįsta anamnezės, neurologinio ištyrimo duomenimis, smegenų skysčio tyrimu ir elektroneuromiografiija. Retais atvejais GBS gali pasireikšti kartu su kitais sindromais. Užpakalinės grįžtamosios encefalopatijos sindromas (PRES) – tai klinikinis-radiologinis sindromas, apibrėžiamas būdingais klinikiniais ir radiologiniais pakitimais. Šių sindromų pasireiškimui įtakos gali turėti įgimtas Klipelio-Feilio (Klippel-Feil) sindromas (KFS), kuris diagnozuojamas, kai vaizdiniuose tyrimuose matomas bent dviejų stuburo kaklinės dalies slankstelių susijungimas. Šis sindromas gali lemti stuburo kanalo stenozę, kaklo mobilumo sumažėjimą, žemesnę plaukų augimo liniją. Šiame straipsnyje pristatome 72 metų pacientės, kuri kreipėsi į Skubios pagalbos skyrių dėl sutrikusios kalbos, dezorientacijos, atvejį. Neurologinio ištyrimo duomenys buvo būdingi encefalopatijai. Pacientei buvo atlikti išsamūs diagnostiniai tyrimai ir, vertinant gautus duomenis, patvirtinta diagnozė – GBS, kai pasireiškia PRES ir KFS. Šis atvejis parodo GBS klinikinių simptomų įvairovę ir išsamios diferencinės diagnostikos svarbą pacientams, tiriamiems dėl demielinizuojančios polineuropatijos.
Raktažodžiai: Guillaino-Barré sindromas, užpakalinės grįžtamosios encefalopatijos sindromas, Klippelio-Feilio sindromas, demielinizuojanti polineuropatija.
________
* Address: Gabrielė Jankauskaitė, Faculty of Medicine, Lithuanian University of Health Sciences, Eivenių 2, LT-50009, Lithuania.
E-mail: gabriele.jankauskaite@stud.lsmu.lt
Received: 29/11/2024. Accepted: 11/02/2025
Copyright © Gabrielė Jankauskaitė, Gintarė Žemgulytė, Renata Balnytė, 2024. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Guillain-Barré Syndrome (GBS) is an acute, immune-mediated peripheral nervous system disorder characterized by immune system damage to neurons. This leads to acute, progressive, and symmetric limb weakness, along with the reduction or absence of tendon reflexes and dysautonomia [1]. The condition is more prevalent in males than females, with a male-to-female ratio of 3:2, and it affects the population with an incidence of 1–2 cases per 100 000 person-years. Before the onset of GBS, symptoms of a respiratory or gastrointestinal tract infection are reported by two-thirds of patients. Among infectious triggers, Campylobacter jejuni is a leading cause, with other pathogens such as cytomegalovirus, Epstein-Barr virus, Varicella zoster virus, Mycoplasma pneumoniae, and Haemophilus influenzae also implicated [2].
GBS can occur concurrently with other syndromes, which complicates its diagnosis. One of the syndromes described in this case study is Posterior Reversible Encephalopathy Syndrome (PRES). PRES is a rare clinical-radiological syndrome characterized by seizures, headaches, altered mental status and visual disturbances, often accompanied by fluctuating blood pressure. Typical brain Magnetic Resonance Imaging (MRI) findings include subcortical vasogenic edema, particularly in the occipital and parietal lobes. Hypertension is often defined as a common feature of PRES. PRES can be observed in a variety of disorders and underlying conditions, including eclampsia, solid organ and bone marrow transplants, exposure to various immunosuppressants and cytotoxic drugs, as well as systemic auto-inflammatory diseases with a compromised kidney function, such as systemic lupus erythematosus. Clinically, the syndrome is manifested by headache, nausea and vomiting, altered mental status, decreased alertness, convulsions and visual disturbances [3].
There is growing evidence suggesting a possible correlation between Klippel-Feil Syndrome (KFS) and the development of PRES. KFS is a congenital syndrome resulting from the fusion of at least two cervical vertebrae. Despite this clinical and genetic heterogeneity, it has been reported that the most frequent cervical fusions occur at C5–C6 and C2–C3. However, patients with Klippel-Feil syndrome are often asymptomatic; nevertheless, they may develop a number of spontaneous neurologic sequelae as a result of their bony anomalies. KFS is often incidentally detected on cervical imaging following a traumatic event. Its clinical presentation can vary widely, ranging from asymptomatic cases to severe spinal fusion and accompanying visceral abnormalities, necessitating a multidisciplinary approach. Genetic mutations linked to KFS involve chromosomes X, 12, 17, and 22. The syndrome manifests with restricted cervical spine mobility, a shortened neck, and a low posterior hairline. Complications such as central canal stenosis may also arise. In some cases, KFS is associated with multisystem involvement, including cardiac, renal, and auditory abnormalities [4,5,6].
This clinical case report examines the association between GBS, PRES, and KFS.
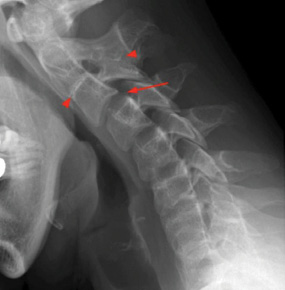
A 72-year-old female patient arrived at the Emergency Department (ED) of the Hospital of Lithuanian University of Health Sciences Kaunas Clinics. The patient presented with complaints of speech disturbances, neck pain, numbness in her hands, and difficulty climbing stairs. The symptoms had persisted for over two days without improvement. Based on her medical history, in April 2023, the patient had been treated for a severe episode of neck pain, and a neck X-ray was performed, revealing congenital partial fusion of the C2–C3 vertebrae, as indicated by arrows in Figure 1.

The patient was consulted by a neurologist, laboratory tests were conducted, and hypertensive encephalopathy was suspected. She was discharged with recommendations for further outpatient treatment. In the course of a week, according to the patient’s husband, her condition worsened. The patient’s overall condition deteriorated, accompanied by progressive muscle weakness. She experienced a generalized tonic-clonic seizure and was evaluated in the ED due to her worsening clinical status. Upon examination, the patient was found to be disoriented, which made her physical examination challenging. The patient underwent brain computed tomography, brain and neck computed tomography angiography, and a lumbar puncture. Detailed patient complaints, neurological assessment, laboratory findings, and instrumental examination results are summarized in Tables 1 and 2.
Given the unclear diagnosis, the patient was hospitalized in the Neurology Department for further evaluation and management. Following hospitalization, the patient’s level of consciousness improved dynamically, though limb weakness and absence of tendon reflexes persisted. A detailed neurological assessment is provided in Table 1. Based on the CSF findings of elevated protein levels, acute inflammatory polyneuropathy was suspected, and ENMG was performed. The results indicated nervus medianus dextra sensory fibers axonopathy, sensory and motor fibers focal demyelination in the carpal tunnel area and signs of motor fibers desynchronization, conduction disorder in the proximal parts. Nervus ulnaris dextra, nervus radialis dextra motor fibers with partial conduction blocks in the proximal parts were detected. Nervus tibialis, nervus peroneus signs of motor fiber conduction disorder in proximal parts in both sides were observed. Prolonged distal latency of motor nerve responses was also noted. Acute denervation in the examined limb muscles was not observed. ENMG findings were not inconsistent with GBS. Based on clinical examination, laboratory and imaging findings, the clinical diagnosis of GBS was made. The patient was treated for acute demyelinating polyneuropathy with intravenous immunoglobulin (IVIG) at a dose of 2g/kg daily for 5 days.
|
Department |
Date |
Symptoms |
Objectively examination |
|
LSMU KK Emergency Department |
Oct 03 2023 |
Impaired speech, numbness of arms and difficulty climbing stairs. |
Without focal neurological deficits. BP – 218/105mmHg. |
|
LSMU KK Emergency Department |
Oct 09 2023 |
Impaired speech, disorientation, numbness of arms and limb weakness (symptom clarification post-GTCS). |
Disoriented, does not maintain meaningful contact, speaks in single slurred words, resists eye-opening, Patellar and tendon reflexes not elicited. Unable to hold legs in a bent position. Neck stiffness present. BP – 158/99mmHg. |
|
LSMU KK Neurology Department |
Oct 10 2023 |
Weakness of arms and legs, numbness of arms. |
Conscious, oriented to place and person, disoriented in time. Cranial nerves intact. Muscle strength examination revealed 2/5 strength proximally and 2-3/5 strength distally in both arms and 1/5 strength proximally and 2-3/5 strength distally in both legs. Tendon reflexes (-). No pathological reflexes detected. BP – 140/90mmHg. |
|
LSMU KK Neurology Department |
Oct 24 2023 |
Generalized weakness, inability to defecate. |
Conscious, communicative, oriented. Speech normal. Cranial nerves intact. Muscle strength: 3-4/5 proximally and 4/5 distally in arms (can hold arms raised). 2-3/5 proximally and 2/5 distally in legs. Reports paresthesia’s in legs. Tendon reflexes (-). No pathological reflexes. Moves within bed. BP – 120/82mmHg. |
|
Department |
Laboratory |
Instrumental |
|
LSMU KK Emergency Department (Oct 03 2023) |
Blood tests: Routine peripheral blood test and blood biochemistry were normal. Sodium level 137mmol/l. |
Not performed. |
|
LSMU KK Emergency Department (Oct 09 2023) |
Blood tests: Leukocytosis (12.4 x 109/l), hyponatremia (129mmol/l). CSF: Clear, cytology – 0, total protein – 1.06 g/l. |
Brain CT and brain/neck CTA – no pathological lesions detected. |
|
LSMU KK Neurology Department (Oct 10 to Oct 24, 2023) |
Blood tests: Peripheral blood test – no abnormalities. Blood biochemistry: Sodium levels fluctuated between 128–139mmol/l, CRP ranged from 12.6 to 55.3mmol/l. Vitamin B1 – 258nmol/l (66.5–200), B6 – 84.4nmol/l (35–110), B12 – 618 pmol/l (127–517). Testing for B. burgdorferi IgM and IgG, tick-borne encephalitis antibodies, paraneoplastic syndrome panel, antibodies against neuronal surface antigens in blood and CSF, antibodies against neuronal antigen – negative. CMV and EBV IgM, HIV-1/HIV-2 antibodies, and p24 antigen – negative. CMV IgG – 1659.4 IU/ml (0–1) EBV IgG – 200 IU/ml (0–20). |
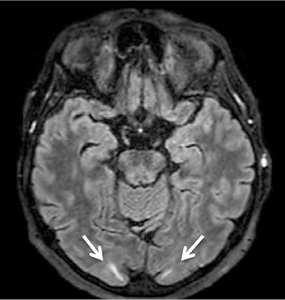
CT with venography, chest X-ray, abdominal US – no abnormalities. Awake EEG – no clear specific epileptiform activity observed. Sleep EEG (13th and 19th day) – bilaterally temporally, asynchronously connected, without a clear, stable dominant lateralization, single epileptiform potentials with peak wave morphology and slow (theta-delta) range episodes were recorded. Brain MRI – in bilateral occipital lobes, subcortically small hyperintense areas in T2W – mild PRES findings (Figure 2). |
On the third day of hospitalization, the patient experienced visual hallucinations and two additional generalized tonic-clonic seizures. MRI results revealed small subcortical hyperintense areas in the bilateral occipital lobes on T2-weighted images, which were moderately suggestive of PRES (Figure 2 and Table 2). There was no evidence of renal insufficiency, eclampsia, sepsis, allergies, autoimmune diseases, or the use of immunosuppressive or cytotoxic drugs. After managing the patient’s blood pressure, her condition began to improve: the visual hallucinations regressed, and no further seizure episodes occurred. Following treatment for GBS, muscle strength in the arms and legs improved. The patient was able to eat independently by using her left hand and sit up in bed.
After 15 days at the Department of Neurology, the patient was transferred to the Department of Neurorehabilitation for further treatment. At the end of the period for rehabilitation, the patient was able to move and walk independently on flat surfaces but required supervision or assistance when climbing stairs, walking uphill or downhill, or walking on uneven surfaces. The patient’s independence, as assessed by the Barthel index, improved, and she was discharged home, referring to the care of the family doctor.
The simultaneous occurrence of GBS and PRES with KFS in individuals is a rare entity. Presently, the connection between these syndromes is not firmly established. Upon reviewing the associations between GBS and PRES, it is hypothesized that dysautonomia associated with GBS can lead to abrupt fluctuations in the blood pressure and activation of the sympathetic nervous system, which may trigger PRES [7]. Several potential mechanisms have been discussed in the literature. It has been described that elevated systemic blood pressure can impair the autoregulation of cerebral blood flow, leading to an increase in the cerebral mean arterial pressure. This can lead to hyperperfusion, an increased capillary hydrostatic pressure, vasodilatation, and vasogenic edema. Furthermore, elevated cerebral arterial pressure may disrupt the integrity of the physiological blood-brain barrier, allowing plasma extravasation through the tight junctions of the brain parenchyma, potentially leading to cerebral edema.
Another possible mechanism highlights the importance of the immune system through T lymphocyte activation, cytokine release, increased endothelial permeability, and vasogenic edema. The activation of endothelial cells, accompanied by the release of mediators – such as histamine, free radicals, bradykinin, and arachidonic acid – results in blood-brain barrier dysfunction, leading to cerebral edema [3,8,9].
KFS in conjunction with GBS has only rarely been documented in the literature. It is proposed that the abnormalities in the renal, cardiac, and vascular systems associated with KFS (such as ventricular septal defects, aortic coarctation, hypoplastic aortic arch, aortic root aneurysm, and abnormal pulmonary vasculature) may influence fluctuations in the arterial blood pressure and thereby contribute to the development of PRES [10].
As previously mentioned, GBS is one of the risk factors associated with PRES. The manifestations of PRES include headache, encephalopathy, visual disturbances, and seizures. In the presented case, when the patient was first admitted to the hospital, elevated blood pressure was observed, although the medical history did not reveal any prior hypertension. On the second occasion, high blood pressure was observed concurrently with generalized tonic-clonic seizures. In this case, acute moderate hyponatremia was detected, which may have contributed to confusion; however, epileptic seizures are not characteristic of moderate hyponatremia [11,12]. Furthermore, the patient’s hyponatremia was managed on October 12, at which time the serum sodium concentration was recorded at 130 mmol/l. After correcting the sodium levels, a brain MRI was performed, revealing findings consistent with PRES. Imaging studies of the brain and radiological evaluation are crucial for diagnosing PRES.
Following the diagnosis of GBS, IVIG was administered, and the patient experienced visual hallucinations. It is known that IVIG administration can lead to hyperviscosity, hypercoagulability, and platelet hyperactivity, which in turn may contribute to the development of PRES. Some features of this syndrome (seizures, cerebral edema) were observed before IVIG treatment, but it is important to note that visual hallucinations developed only after IVIG administration [13,14].
As mentioned above, GBS can be triggered by various infections. In this case, the patient received the ‘Influvac Tetra’ influenza vaccine (G22 series) on September 20 and was admitted to the hospital on October 3. The literature describes cases where influenza vaccination may increase the risk of GBS due to an inadequate immune response. It is also associated with an increased risk of GBS within the first month following vaccination. However, only 1.5% of GBS cases in Denmark are linked to the influenza vaccine [15].
The simultaneous occurrence of GBS, PRES, and KFS in a single patient is a rare case. The pathogenesis mechanisms of these syndromes at certain stages of the disease may influence symptom manifestation and trigger immune and inflammatory responses. As a result, the dysautonomia induced by GBS and the IVIG treatment likely provoked PRES. KFS may have predisposed to the development and manifestation of complications. Therefore, it is crucial in the clinical practice to consider the potential effects of neurological syndromes on other body systems, as their dysfunction can exacerbate the neurological condition.