
Neurologijos seminarai ISSN ISSN 1392-3064 / eISSN 2424-5917
2024, 28(100), pp.108–117 DOI: https://doi.org/10.15388/NS.2024.28.100.4

Gabija Gurskaitė
Faculty of Medicine, Vilnius University, Vilnius, Lithuania
E-mail: gabija.gurskaite@mf.stud.vu.lt
Rūta Samaitienė Aleknienė*
Clinic of Children’s Diseases; Faculty of Medicine, Vilnius University, Vilnius, Lithuania;
E-mail: ruta.samaitiene@mf.vu.lt
Abstract. Background and Objectives: Subacute Sclerosing Panencephalitis (SSPE) is a rare, progressive, and fatal neurological disorder caused by persistent measles virus infection, predominantly affecting children. The recent resurgence of measles globally, driven by declining vaccination rates, has highlighted the need for effective SSPE management strategies. Currently, treatment approaches focus on managing symptoms and slowing down the disease progression. This review aims to systematically evaluate treatment outcomes for SSPE based on the disease stage at which the therapy is initiated. Materials and Methods: A comprehensive PubMed search identified publications from 1994 to 2024 with the objective to list studies covering in detail SSPE treatment regimens and reporting on patient outcomes by the disease stage at treatment initiation. The inclusion criteria were as follows: randomized controlled trials, cohort studies, and case reports which clearly documented the treatment initiation stages and outcomes. The review classified cases into six treatment groups based on the most used medication combinations for the early (stages I–II) and advanced stages (stages III–IV) of SSPE. Results: A total of 30 studies comprising 80 SSPE cases met the inclusion criteria. Ribavirin combined with interferon and Isoprinosine showed favorable outcomes, particularly in early-stage patients. In contrast, Isoprinosine monotherapy resulted in the highest progression rates across both stages. Other combinations demonstrated varied effectiveness. Antiepileptic drugs provided symptomatic relief but did not alter the disease progression. Conclusions: This systematic review highlights the influence of the SSPE treatment initiation stage on the patient outcomes, suggesting that a tailored approach based on the disease progression may improve the treatment efficacy.
Keywords: Subacute sclerosing panencephalitis; SSPE; measles infection; SSPE staging; pediatric neurology.
Santrauka. Poūmis sklerozuojantis panencefalitas (PSPE) yra reta, progresuojanti mirtina neurologinė liga, kurią sukelia tymų viruso infekcija, dažniausiai paveikianti vaikus. Pastaruoju metu pasaulyje matomas tymų atvejų skaičiaus didėjimas, susijęs su mažėjančiomis skiepijimo aprėptimis, pabrėžia veiksmingų PSPE gydymo strategijų poreikį. Dabartiniai gydymo metodai orientuoti į simptomų kontrolę ir ligos progresavimo lėtinimą. Šios sisteminės literatūros apžvalgos tikslas – įvertinti skirtingų PSPE gydymo strategijų efektyvumą, atsižvelgiant į tai, kurioje ligos stadijoje pradedama terapija. Peržiūrėtos 1994–2024 metų „PubMed“ publikacijos, kuriose aprašomi PSPE gydymo būdai ir pacientų išeitys, priklausomai nuo gydymo pradžios stadijos. Į analizę įtraukti atsitiktinių imčių kontroliuojami tyrimai (angl. RCT), kohortiniai tyrimai ir atvejų, kai aiškiai dokumentuota gydymo pradžios stadija ir rezultatai, aprašymai. Atvejai buvo suskirstyti į šešias grupes pagal dažniausiai vartojamų vaistų derinius ankstyvomis (I–II) ir pažengusiomis (III–IV) PSPE stadijomis. Analizuoti 30 publikacijų duomenys, apimantys 80 atvejų. Ribavirino ir interferono derinys su isoprinozinu pasižymėjo palankiais rezultatais, ypač gydant pacientus, kuriems buvo ankstyvosios ligos stadijos. O štai isoprinozino monoterapija buvo susijusi su didžiausiu ligos progresavimo dažniu visose stadijose. Kiti vaistų deriniai pasižymėjo varijuojančiu veiksmingumu. Nors priešepilepsiniai vaistai palengvino simptomus, jie nesulėtino ligos progresavimo. Ši sisteminė apžvalga pabrėžia PSPE gydymo pradžios stadijos įtaką pacientų išgyvenamumui ir leidžia daryti prielaidą, kad individualizuotas požiūris, atsižvelgiant į ligos progresavimą, gali pagerinti gydymo efektyvumą.
Raktažodžiai: poūmis sklerozuojantis panencefalitas, PSPE, tymai, vaikų neurologija.
________
* Address: Rūta Samaitienė Aleknienė, Clinic of Children’s Diseases; Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
E-mail: ruta.samaitiene@mf.vu.lt
Received: 25/01/2025. Accepted: 30/03/2025
Copyright © Gabija Gurskaitė, Rūta Samaitienė Aleknienė, 2024. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Subacute Sclerosing Panencephalitis (SSPE) is a progressive and fatal neurological disorder resulting from a persistent measles virus infection, mainly affecting the paediatric population. SSPE occurs in approximately 6.5 to 11 cases per 100,000 measles infections, predominantly affecting children under five years old [1]. Recent epidemiological data underscore the resurgence of measles globally. In 2022, approximately 9 million cases of measles were reported worldwide, on top of over 300,000 confirmed cases in 2023 [2,3]. The resurgence of measles, driven by the declining immunization rates and inadequate vaccine coverage, significantly elevates the risk of SSPE, particularly in unvaccinated and under-vaccinated pediatric populations.
SSPE is characterized by cognitive decline, seizures, and eventual coma [4]. Currently, there is no cure for SSPE, and no standardized treatment protocol has been established. The available therapies primarily focus on symptom management and slowing down the disease progression [5].
Isoprinosine, an antiviral drug with immunomodulatory effects, is widely used for treating SSPE. Although Isoprinosine is known for its immunomodulatory properties, it has demonstrated only limited success [6]. Interferons are naturally produced by animal cells in response to viral infections, aiming to inhibit viral replication, activate immune responses, and are also used in treating SSPE in both monotherapy and in addition with Isoprinosine [5]. Ribavirin, another antiviral medication, was found to inhibit the replication of SSPE virus strains in in vitro studies and animal models [7]. Lamivudine, an antiretroviral typically used in HIV treatment, has also been used in SSPE due to its impact on viral RNA synthesis [8,9]. Antiepileptic Drugs (AEDs) are primarily used to manage seizures in SSPE, providing symptomatic relief [1].
No systematic review has yet evaluated the impact of the SSPE stage at treatment initiation on the therapeutic outcomes.
This review aims to analyse the effectiveness of various treatment types in SSPE patients by comparing the outcomes (improvement/stabilization and progression) based on the stage at which the treatment is initiated (early stages I–II vs. advanced stages III–IV).
The review includes patients diagnosed with Subacute Sclerosing Panencephalitis (SSPE) and considers studies which clearly describe SSPE treatment regimens and specify the stage of SSPE before treatment initiation, as well as the treatment outcomes. A comprehensive search was conducted by using PubMed. The search was performed on March 1, 2024. The filters included ‘English’, ‘Full text’, and ‘Abstract’, with a publication time frame from 1994 to 2024. The strategy ensured the inclusion of a wide range of relevant studies. A PRISMA flow chart illustrates the selection process (Figure 1).
The inclusion criteria were as follows: 1) Randomized Controlled Trials (RCTs), cohort studies, case-control studies, and case reports; 2) studies that specify both the treatment initiation stage and the treatment outcomes and use well-defined clinical staging systems; 3) in terms of publications where not all patients had clearly documented stages or outcomes, only cases with sufficient data were included.
Exclusion criteria: 1) patients whose treatment initiation stage and/or treatment outcomes are not specified or defined, i.e., wherever an unclear staging system was used; 2) immunocompromised and/or pregnant patients; 3) studies where the patients did not receive specified SSPE treatments; 4) literature reviews, systematic reviews, and meta-analyses (these studies are excluded to ensure the inclusion of primary research data and direct clinical evidence).
Publications were not filtered by location, hospital settings, income, or similar factors, thus aiming to include a wide range of publications to thoroughly assess the treatment outcomes.

Cases were classified to assess the effectiveness of different treatments at different stages of the disease progression. The classification was based on:
1. SSPE stage before and after treatment: cases were divided into two groups: patients that started treatment at the early stages (I–II) and the advanced stages (III–IV).
2. Treatment outcomes: the outcomes were divided into two groups: improvement/stabilization versus progression.
3. Different treatment types: cases were classified into six treatment groups based on the most used medication combinations: Isoprinosine monotherapy, Interferon ((IFN-α) or IFN-β) with Isoprinosine, Interferon monotherapy, Ribavirin with Interferon and Isoprinosine, Lamivudine with Interferon and Isoprinosine, and Antiepileptic Drugs (AEDs) as monotherapy. IFN-α and IFN-β were grouped for analysis, as some studies did not differentiate between them. In cases where only one type of Interferon was used, it was still included in the combined interferon category to allow for broader comparison. One case involving intravenous gamma globulin treatment was excluded due to insufficient data.
The protocol for this systematic review was registered and published on PROSPERO (ID: CRD42024547588).
We included 30 publications and 80 cases meeting the predefined inclusion criteria. Figure 2 highlights six different treatment approaches and the percentage of patients who either improved/stabilized or progressed under each treatment type across both stages. For detailed information on patient outcomes by the treatment type and disease stage, see Appendix A, Table 1.
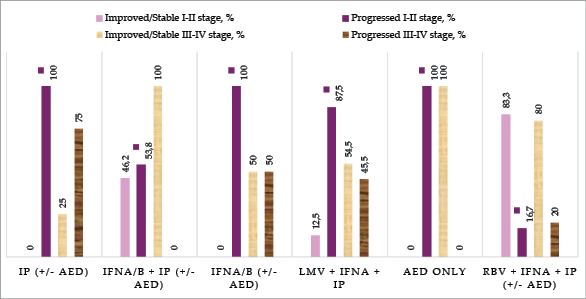
Isoprinosine monotherapy showed the poorest outcomes, with all patients in the early stages (I–II) and 75% of patients in the advanced stages (III–IV) experiencing disease progression.
A combination of Interferon (IFN) with Isoprinosine led to mixed outcomes. In the early stages, 46.2% of patients improved or stabilized, while 53.8% progressed. However, in the advanced stages, this combination seemed to be effective, with 100% of patients improving or stabilizing. It is important to note that only 5 patients in stages III–IV received this treatment, which limits the reliability of the results, as such a small sample size may fail to fully reflect the treatment effectiveness.
Ribavirin combined with IFN and Isoprinosine demonstrated some of the most favorable outcomes, particularly in the early stages, where 83.3% of patients improved or stabilized. In advanced stages, 80% of patients also improved or stabilized. Here, it is also important to note that the sample sizes were small.
Lamivudine combined with IFN and Isoprinosine showed more favorable outcomes in the advanced stages compared to the early stages, with 54.5% of advanced-stage patients improving or stabilizing, versus only 12.5% in early stages.
Antiepileptic drugs used as monotherapy were observed in only three cases, making definitive conclusions difficult. In the early stages, all patients progressed, while one patient in the advanced stages improved.
Interferon monotherapy also had limited data, with only one patient improving, while the remaining two patients (one from each stage) progressed.
In our analysis, a Ribavirin, IFN and Isoprinosine combination therapy showed the lowest overall progression rates, indicating potential efficacy in slowing down the disease progression. Our findings align with the results of another publication which reported clear improvements particularly in patients treated during the early stages of SSPE [7]. Gutierrez et al. found conflicting results in the literature: while some studies showed that Ribavirin combined with Interferon provides better outcomes than monotherapy, there are also reports suggesting that such a combination therapy can be totally ineffective [11].
Isoprinosine monotherapy in our study exhibited the highest progression rates across all stages, thus echoing findings by Pritha et al. [5]. Jafri et al. also agree on Isoprinosine’s limited success and potential adverse effects [12]. Hoppen et al. state that larger case series suggest a potential benefit of Isoprinosine monotherapy, but no conclusive evidence exists [13].
According to Samia et al., IFN and Isoprinosine combination therapy has been reported to induce remission or stabilize 44–55% of SSPE cases [14]. These results are similar to our findings, where approximately half of the patients who received Interferon-alpha and Isoprinosine experienced improvement or stabilisation. However, the improvement is often temporary, as long-term follow-up reveals eventual neurological deterioration [15]. Studies comparing Isoprinosine alone to its combination with intraventricular IFN-α show no significant difference in the improvement rates [16,17]. In contrast, in a study done in 1998, subcutaneous IFN-β was found to extend the survival and delay the disease progression when used with Isoprinosine for five out of seven patients [18]. Despite these benefits, the overall effectiveness of interferons in SSPE treatment remains limited by the temporary nature of remission and the potential side effects.
Despite these varied treatment outcomes observed across different therapies, the overall prognosis for SSPE remains challenging, with the majority of patients failing to survive beyond five years from the onset [19].
Given the rarity of SSPE, most studies included in this review consist of small patient cohorts or case reports, which can affect the accuracy and generalizability of the results. Reliance on smaller studies raises the risk of a publication bias, where positive or more remarkable outcomes are more likely to be published than negative or inconclusive results. Consequently, the perceived efficacy of certain treatments may be overestimated due to the underreporting of the less favorable outcomes. The absence of large-scale randomized controlled trials further limits the strength of the conclusions drawn from this review, thus emphasizing the need for caution when interpreting the treatment effectiveness.
Future research should aim to include larger studies with the objective to diminish these biases and provide a more comprehensive understanding of the SSPE treatment outcomes.
The results highlight the variability in the treatment outcomes for SSPE based on both the disease stage and the therapeutic regimen. Ribavirin combined with IFN and Isoprinosine demonstrated the most favorable results, with high rates of stabilization and improvement in both early and advanced stages, but the sample sizes were small. The Isoprinosine monotherapy resulted in the poorest outcomes, with high progression rates regardless of the timestamp when the treatment was initiated. Monotherapies, including Interferon and antiepileptic drugs, demonstrated minimal efficacy. Other combinations, such as Lamivudine or IFN with Isoprinosine, showed more mixed outcomes but were more effective in the advanced stages. While the search for more effective treatments continues, personalized management and measles vaccination programs remain the most reliable strategies to prevent the primary infection and reduce the future cases of SSPE.
Supplementary Materials: Supplementary Table S1: Overview of SSPE Cases and Treatment Outcomes. This table can be accessed at: https://figshare.com/s/057139ebb01f2fe2144b
Author Contributions: Conceptualization: R.S.A; methodology: R.S.A, G.G; formal analysis: G.G., R.S.A.; data curation: G.G, R.S.A.; writing – original draft preparation: G.G.; writing – review and editing: R.S.A.; visualization: G.G., R.S.A. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Informed Consent Statement: Not applicable.
Data Availability Statement: No new data were created or analyzed in this study. All data used in this review are available within the cited publications in the reference list. The combined dataset of the SSPE cases used in this analysis is available in the supplementary materials (Supplementary Table S1).
Acknowledgments: The authors received no additional support for this work.
Conflicts of Interest: The authors declare no conflict of interest.
Table 1 provides a summary of the patient outcomes categorized by the type of treatment and the stage of disease progression. It includes details on improvement, stabilization, and progression rates across early and advanced stages of SSPE. For detailed case information, see Supplementary Table S1 [9,15,18,20–46].
|
Stages I–II |
Stages III–IV |
|||||
|
Improved or stable |
Progressed |
Total |
Improved or stable |
Progressed |
Total |
|
|
IP (+/- AED) [20–25] |
0 |
9 |
9 |
1 |
3 |
4* |
|
IFNα/β + IP (+/- AED) |
12 |
14 |
26 |
5 |
0 |
5* |
|
IFNα/β (+/- AED) [34–36] |
0 |
1 |
1* |
1 |
1 |
2* |
|
LMV + IFNα + IP [9] |
1 |
7 |
8 |
6 |
5 |
11 |
|
AED only [43,44] |
0 |
2 |
2* |
1 |
0 |
1* |
|
RBV + IFNα + IP (+/- AED) |
5 |
1 |
6 |
4 |
1 |
5* |