Neurologijos seminarai ISSN ISSN 1392-3064 / eISSN 2424-5917
2024, 28(100), pp. 100–107 DOI: https://doi.org/10.15388/NS.2024.28.100.3
Apžvalginis mokslinis straipsnis / Review Article
Lukas Kalytis*
Vilniaus universitetas, Medicinos fakultetas
Jūratė Dementavičienė
VULSK, Radiologijos ir branduolinės medicinos centras
Summary. Mild traumatic brain injury (mTBI) is a prevalent neurological condition which can result in long-term cognitive and functional impairments, often linked to microhemorrhages and subtle bleeding that may not be visible when using the conventional imaging techniques, such as CT or standard MRI. Susceptibility Weighted Imaging (SWI) has emerged as a valuable tool for detecting these subtle abnormalities due to its enhanced sensitivity to magnetic susceptibility differences. This literature review explores the role of SWI in identifying microhemorrhages associated with mTBI. It examines the advantages of SWI over the traditional imaging modalities, highlighting its capability to detect microbleeds that are crucial for accurate prognosis of mTBI. Furthermore, the review discusses the integration of SWI with other advanced imaging techniques, such as Quantitative Susceptibility Mapping (QSM), to improve diagnostic accuracy and monitor imaging changes over time.
Keywords: Susceptibility weighted imaging (SWI), mild traumatic brain injury (mTBI), microhemorrhages cerebral microbleeds (CMBs), traumatic microbleeds (TMBs), quantitative susceptibility mapping (QSM), post-concussive syndrome (PPCS).
Santrauka. Nedidelis trauminis smegenų sužalojimas (mTBI) yra neurologinė būklė, galinti sukelti ilgalaikius kognityvinius ir funkcinius sutrikimus, kurie dažnai susiję su mikrohemoragijomis ir negausiu kraujavimu, kurio negalima nustatyti naudojant įprastus vaizdinimo metodus, tokius kaip kompiuterinė tomografija (KT) ar standartinė magnetinio rezonanso tomografija (MRT). Magnetinio jautrumo vaizdinimas (SWI) tapo vertingu įrankiu aptinkant šiuos subtilius pakitimus dėl savo gebėjimo aptikti magnetinio jautrumo skirtumus. Ši literatūros apžvalga nagrinėja SWI vaidmenį nustatant su mTBI susijusias mikrohemoragijas. Joje analizuojami SWI pranašumai, palyginti su tradiciniais vaizdinimo metodais, pabrėžiant šio metodo gebėjimą aptikti mikrohemoragijas, kurios yra labai svarbios tiksliai mTBI prognozei nustatyti. Be to, apžvalgoje aptariama SWI integracija su kitais pažangiais vaizdinimo metodais, tokiais kaip kiekybinis jautrumo žemėlapis (QSM), siekiant pagerinti diagnostinį tikslumą ir sekti vaizdų pokyčius laikui bėgant.
Raktažodžiai: magnetinio jautrumo vaizdinimas (SWI), nedidelis trauminis smegenų sužalojimas (mTBI), mikrohemoragijos, smegenų mikrohemoragijos (CMBs), trauminis mikrokraujavimas (TMB), kiekybinis jautrumo žemėlapis (QSM), potrauminis sindromas (PPCS).
_________
* Adresas: Lukas Kalytis, Vilniaus universitetas, Medicinos fakultetas. Rudens gatvė 32–2, LT-01214 Vilnius. Tel. + 370 686 53 026, el. paštas lukas.kalytis@mf.stud.vu.lt
Received: 07/11/2024. Accepted: 12/01/2025
Copyright © Lukas Kalytis, Jūratė Dementavičienė, 2024. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
To examine the scientific literature and discuss the role of SWI in identifying microhemorrhages in mTBI patients.
A literature review was conducted using the medical databases PubMed and Google Scholar. The search utilized the following keywords: Susceptibility Weighted Imaging (SWI), Mild Traumatic Brain Injury (mTBI), Microhemorrhages, Cerebral microbleeds (CMBs), Quantitative Susceptibility Mapping (QSM), post-concussive syndrome (PPCS). Publications relevant to the topic and written in English were selected. Based on all the applied criteria, this literature review is based on 18 scientific articles published between 2003 and 2023.
Traumatic brain injury (TBI) is a major source of health loss and disability worldwide. Globally, the annual incidence of TBI is variably estimated at 27 to 69 million [1]. The large majority of traumatic brain injuries (70–90%) can be classified as mild traumatic brain injury (mTBI), which is indicated by a Glasgow Coma Scale (GCS) score between 13 and 15 on admission to the emergency department, loss of consciousness <30 min, and posttraumatic amnesia <24 h. Patients who have experienced a mild traumatic brain injury (mTBI) may show normal brain computed tomography (CT) scans, yet still exhibit various symptoms. These can include physical, mental, and sensory disturbances such as headaches, dizziness, difficulty concentrating, sleep problems, and mood changes. While most individuals recover within weeks, a significant minority – between 10% and 20% – experience symptoms persisting beyond three months; this condition is termed persistent post-concussive syndrome (PPCS) [2,3]. This prolonged condition can significantly impact an individual’s well-being, potentially leading to mental health issues and difficulties in social functioning. Given the potential long-term effects of mild traumatic brain injury (mTBI), it is essential to conduct comprehensive brain evaluations by using advanced imaging techniques, even when the initial CT scans appear normal. Traumatic cerebral microbleeds (CMBs) are an important prognostic factor which can indicate poor outcomes in patients with mTBI. Among MRI sequences, Susceptibility Weighted Imaging (SWI) has proven to be the most effective for detecting these microbleeds. SWI enhances the sensitivity to microbleeds, while providing additional information beyond that obtained from the conventional MRI and CT scans, thus offering a more detailed and comprehensive assessment of brain injuries.
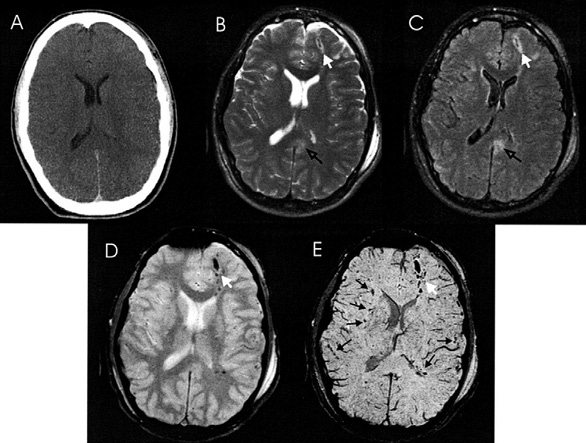
Computed tomography (CT) remains the cornerstone imaging modality for evaluating patients with a head trauma, primarily due to its rapid availability and effectiveness in identifying life-threatening intracranial hemorrhages. Non-contrast CT scans are especially crucial in the acute setting, as they allow radiologists to quickly identify and assess critical structural abnormalities such as skull fractures, epidural and subdural hematomas, subarachnoid hemorrhages, and brain contusions. Additionally, CT is effective in detecting signs of an elevated intracranial pressure, such as a midline shift or compression of ventricular and cisternal spaces. These findings are vital for determining the need for an immediate neurosurgical intervention and for predicting clinical outcomes, as well as for guiding timely and appropriate patient management [4]. However, CT scans offer limited information in cases of mTBI, with fewer than 10% of patients showing detectable abnormalities on an acute CT scan [5]. Because of that, magnetic resonance imaging (MRI) has been increasingly used as a complementary tool in cases of traumatic brain injury (TBI). MRI offers superior ability to detect and precisely locate smaller hemorrhages and can reveal non-hemorrhagic trauma compared to CT [4,5]. Nevertheless, SWI, as an advanced MRI technique, has shown even greater capability in detecting microbleeds than the conventional MRI.
SWI is a fully velocity-compensated high-resolution 3D gradient-echo sequence which uses magnitude and filtered-phase information, both separately and in combination with each other, to create new sources of contrast. SWI leverages variations in magnetic susceptibility between different tissues, creating phase discrepancies in regions containing paramagnetic deoxygenated blood products, such as deoxyhemoglobin, intracellular methemoglobin, and hemosiderin, compared to the surrounding tissue. The signal-intensity cancellation in the magnitude images and the additional suppression from the SWI filtered-phase images produce a hypointense signal in areas of acute and early subacute hemorrhage [6].


SWI is particularly effective for visualizing cerebral microbleeds due to several key technical advantages [7]. Unlike the two-dimensional (2D) Gradient Recalled Echo (GRE) approach, SWI is acquired as a 3D sequence, allowing for thinner slices and smaller voxel sizes, which enhances the spatial resolution and provides a more detailed view of the brain structures. This enables SWI to detect small lesions and subtle vascular abnormalities that GRE might miss. Furthermore, SWI incorporates flow compensation across all three dimensions (x, y, and z), thereby significantly reducing artifacts from the blood flow and patient movement, resulting in clearer and more accurate imaging. Another major advantage is that SWI captures both magnitude and phase information, allowing these to be processed independently or together to enhance the contrast and detect subtle magnetic susceptibility changes, such as microbleeds or iron deposits. In contrast, GRE does not fully utilize the phase data, making it less effective for identifying such subtle abnormalities [8]. Studies also support the superiority of SWI over GRE, by highlighting its enhanced sensitivity and accuracy in detecting subtle brain abnormalities. Research conducted by Tong et al. [9] and Babikian et al. [10] has demonstrated that SWI is three to six times more effective than the standard T2*-weighted gradient-echo sequences in visualizing the size, number, volume, and distribution of hemorrhagic lesions. Furthermore, it is observed that microbleeds could be detected long time after the injury and remain unchanged for at least 100 days following the injury on in vivo MRI scans [11]. However, in the acute phase, microbleeds are not static, but, instead, they change in number and volume over time. Consequently, the timing of imaging is essential for optimizing the prognostic value of SWI. The distinct biophysical characteristics of microbleeds lead to a temporary alignment in intensity with the surrounding white matter on imaging, which can obscure their visibility within 24 to 72 hours after the injury. This temporary decrease in detectability underscores the necessity of accounting for the time elapsed since the trauma when interpreting SWI findings. During this period, an apparent absence or reduction in the number of microbleeds may not accurately represent the true extent of the injury [12].
However, SWI has several certain limitations. It is not effective on its own for determining the age of lesions, as both acute and chronic blood products appear as signal loss on T2-weighted imaging. Additionally, SWI only offers indirect evidence of axonal injury through microvascular shear damage [5]. It is crucial to interpret traumatic microbleeds as signs of traumatic microvascular injury and not only as axonal injury. And, for this purpose, evaluating mTBI by using a combination of SWI and diffusion tensor imaging (DTI) may provide further evidence of microstructural brain abnormalities [13].
CMBs appear as small (2–10 mm in diameter), round or ovoid hypointense foci which exhibit blooming artifacts, thus making them more conspicuous on MRI sequences that are sensitive to susceptibility effects.
When the brain experiences trauma, the rapid acceleration-deceleration movement and rotational forces can stretch and tear small blood vessels. This damage compromises the vessel walls, causing microhemorrhages. These microbleeds indicate a vascular injury and often suggest an axonal injury, as the same forces impact the brain’s axonal networks [11]. Damage to the blood vessels in traumatic brain injury can initiate an inflammatory response, particularly through traumatic microbleeds (TMBs), which are linked to chronic disruption of the blood-brain barrier and delayed microvascular damage. This process leads to toxic iron deposition, persistent neuroinflammation, and axonal demyelination. Additionally, altered blood flow and infiltration of inflammatory cells can result in secondary ischemic brain damage. These interconnected processes of vascular disruption, inflammation, and metabolic changes suggest the potential for long-term complications following mild traumatic brain injuries [11].
In clinical practice, the detection and number of CMBs can create uncertainty for clinicians, as they are also present in asymptomatic individuals, particularly the elderly. The reported prevalence of CMBs in older populations varies based on the average age of the group and the neuroimaging techniques used, with large population studies indicating a prevalence range of 5% to 35% in older adults [14–16]. Given this background prevalence, it becomes crucial to distinguish between traumatic and non-traumatic microbleeds when assessing older patients with a mild traumatic brain injury (mTBI). The most commonly observed types of CMBs are those associated with cerebral amyloid angiopathy (CAA) and hypertensive angiopathy. CAA-related microbleeds are typically lobar, often found in the parietal and occipital lobes, as well as cortical or subcortical areas. In contrast, hypertensive angiopathy is characterized by microbleeds predominantly located infratentorially, within the basal ganglia, thalamus, and periventricular white matter [17].
TMBs are detected in 27% of mild, 47% of moderate, and 58% of severe TBI cases. In contrast to microbleeds associated with small vessel disease or other non-traumatic conditions, traumatic CMBs exhibit distinct characteristics in terms of their location and appearance [17]. These trauma-induced lesions are frequently found in the corpus callosum and at the interface between the grey and white matter. Moreover, traumatic CMBs typically display a more radial and linear pattern, often aligning with the perivascular spaces. This configuration differs noticeably from the rounded shape commonly observed in CMBs linked to small vessel disease (SVD) [17]. These differences in distribution and morphology can aid in distinguishing traumatic from non-traumatic microbleeds in neuroimaging. However, absolute differentiation between these lesions remains challenging.
In a systematic review conducted by Hageman et al. [18], the relationship between microbleeds and persistent posttraumatic symptoms and cognitive performance was examined. The review found no correlation between microbleeds and early symptoms at four weeks. However, patients with a mild traumatic brain injury (mTBI) who had microbleeds exhibited a greater symptom severity at the 12-month mark. In addition, studies compared the cognitive performance of mTBI patients with microbleeds to those without microbleeds, revealing significant differences in the psychomotor speed and the information processing speed at both 3 and 12 months, as well as in the short-term memory (Digit Span) at one month. In contrast, no significant differences were noted in working memory tasks assessed over a period of 3 to 24 months [18]. In addition, both forms of TMBs, punctate and linear, are predictive of the outcome – those with evidence of TMBs were twice as likely to have disability at 30- or 90-days post-injury [11]. The lesion location is a significant factor in predicting the outcomes following mTBI. Wang et al. [19] demonstrated that microhemorrhages in the frontal, parietal, and temporal lobes were associated with an increased risk of developing depression 12 months post-injury – among the major depressive patients, 20 (71.4%) were abnormal on SWI images, whereas, among the 137 non-depressive patients, 12 (9%) were abnormal on SWI images. In a similar study focusing on the chronic phase of mTBI, De Haan et al. [20] identified microbleeds in the frontal and temporal regions; however, they reported that unfavorable functional outcomes were specifically linked to the presence and extent of microhemorrhages in the temporal cortical areas. However, more longitudinal studies are necessary to confirm the predictive value of SWI for post-traumatic complaints and cognitive outcome.
7T MRI, an ultra-high field magnetic resonance imaging technique, shows great potential in the detection of CMBs [17]. This advanced method outperforms lower field strength MRI systems, such as 3T or 1.5T, in both identifying a greater number of CMBs and detecting smaller lesions. The exceptional spatial resolution of 7T MRI enables a more comprehensive examination of CMBs and provides detailed visualization of microvascular structures. However, this technology is not without drawbacks. 7T MRI is more susceptible to imaging artifacts, particularly in regions close to the skull base. These artifacts can potentially limit the technique’s effectiveness in certain areas of the brain, presenting a challenge for widespread clinical application [17].
SWI has several limitations, including its reliance on phase images that may fail to accurately depict local anatomical structures and its sensitivity to the orientation of objects relative to the magnetic field. Interpreting complex regions, such as the basal ganglia, is particularly challenging with SWI due to these constraints. To address these limitations, Quantitative Susceptibility Mapping (QSM) has been proposed as a superior alternative, offering notable advantages, especially in detecting microbleeds in mild traumatic brain injury (mTBI) [21]. Unlike SWI, QSM provides a more precise and quantitative assessment by directly measuring tissue susceptibilities, which improves the accuracy in evaluating the size and concentration of microbleeds. Additionally, QSM minimizes the common SWI artifacts, such as blooming effects, resulting in clearer and more reliable imaging, particularly in complex anatomical regions where SWI may struggle. QSM also enhances contrast sensitivity, allowing for the detection of smaller or more subtle microbleeds that might be missed with SWI. Furthermore, QSM has the ability to differentiate between microbleeds and other brain deposits, like calcifications, ensuring a more accurate diagnosis and effective monitoring of brain injuries [21]. Overall, QSM offers a more advanced and effective approach for the assessment and characterization of microbleeds, supporting improved clinical outcomes and more precise treatment planning.
Furthermore, integrating SWI with other imaging modalities, such as diffusion tensor imaging (DTI) and functional MRI (fMRI), can provide a more comprehensive assessment of mTBI by combining structural, functional, and microstructural insights [13].
1. SWI is one of the most effective methods for identifying microbleeds in mTBI, demonstrating greater sensitivity compared to other conventional MRI sequences, such as T2* imaging.
2. These trauma-induced lesions are often located in the corpus callosum and at the grey-white matter interface, differing from other CMBs by their radial and linear pattern aligned with perivascular spaces.
3. There is some evidence that traumatic microbleeds predict the cognitive outcome and persistent posttraumatic complaints in patients with mTBI.
4. Integration of QSM, fMRI, and DTI with SWI could provide additional valuable information for more comprehensive assessment of patients with mTBI.