Neurologijos seminarai ISSN ISSN 1392-3064 / eISSN 2424-5917
2024, 28(101), pp.171–184 DOI: https://doi.org/10.15388/NS.2024.28.101.4
Apžvalginis mokslinis straipsnis / Review Article
Lukas Kalytis*
Vilnius University, Faculty of Medicine, Vilnius, Lithuania
Jurgita Ušinskienė
Vilnius University, Faculty of Medicine, Vilnius, Lithuania
National Cancer Institute, Department of Diagnostic and Interventional Radiology, Vilnius, Lithuania
Summary. Perineural Spread (PNS) is a critical mechanism of tumor progression in head and neck malignancies, often remaining clinically undetected until the advanced stages. Given its significant implications for treatment planning and patient prognosis, early and accurate diagnosis through radiological assessment is essential. This literature review explores the epidemiology, clinical significance, diagnostic modalities, and, in particular, the radiological characteristics of PNS. Magnetic Resonance Imaging (MRI) remains the gold standard for detecting PNS due to its superior soft tissue contrast and detailed evaluation of nerve involvement. Computed Tomography (CT) and Positron Emission Tomography (PET) serve as complementary imaging modalities, aiding in the assessment of bony changes and metabolic activity, respectively. This review highlights the key radiological features of perineural tumor spread, including nerve enhancement, foraminal enlargement, obliteration of fat planes, and secondary signs such as muscle denervation. Understanding these imaging findings is crucial for accurate staging, guiding therapeutic decisions, and optimizing the patient outcomes.
Keywords: Head-and-neck tumors, perineural tumor spread, perineural invasion, magnetic resonance imaging (MRI) computed tomography (CT), positron emission tomography (PET).
Santrauka. Perineurinis navikų plitimas (PNP) yra kritinis navikų progresavimo galvos ir kaklo piktybiniuose navikuose mechanizmas, dažnai liekantis kliniškai nepastebėtas iki vėlyvųjų stadijų. Atsižvelgiant į didelę įtaką gydymo planavimui ir paciento prognozei, ankstyva ir tiksli diagnostika pasitelkiant radiologinius tyrimo metodus yra būtina. Ši literatūros apžvalga nagrinėja PNP epidemiologiją, klinikinę reikšmę, diagnostikos metodus ir radiologinius požymius. Magnetinio rezonanso tomografija (MRT) tebėra auksinis PNP aptikimo standartas dėl puikios minkštųjų audinių skiriamosios gebos ir išsamios nervų pažeidimo analizės. Kompiuterinė tomografija (KT) ir pozitronų emisijos tomografija (PET) yra papildomi vaizdiniai metodai, padedantys įvertinti kaulų pokyčius ir metabolinį aktyvumą. Šioje apžvalgoje išskiriami pagrindiniai perineurinio plitimo radiologiniai požymiai: kontrasto kaupimasis nervuose, nervų angų prasiplėtimas, riebalinio sluoksnio aplink nervus obliteracija ir antriniai požymiai, tokie kaip raumenų denervacija. Šių radiologinių radinių supratimas yra esminis tiksliai ligos stadijai nustatyti, terapiniams sprendimams priimti ir paciento gydymo rezultatams optimizuoti.
Raktažodžiai: galvos ir kaklo navikai, perineurinis navikų plitimas, perineurinė invazija, magnetinio rezonanso tomografija (MRT), kompiuterinė tomografija (KT), pozitronų emisijos tomografija (PET).
_______
* Address: Lukas Kalytis, Vilnius University, Faculty of Medicine, Rudens St. 32–2, LT-01214 Vilnius.
Tel. + 370 686 53 026. E-mail: lukas.kalytis@mf.stud.vu.lt
Received: 13/03/2025. Accepted: 01/04/2025
Copyright © Lukas Kalytis, Jurgita Ušinskienė, 2024. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The aim of this article is to analyze radiological imaging methods and key radiological features of PNS, supported by illustrative imaging findings from medical practice.
A literature review was conducted by using the medical databases PubMed and Google Scholar. The search utilized the following keywords: Head-and-neck tumors, perineural tumor spread, perineural invasion, Magnetic Resonance Imaging (MRI), Computed Tomography (CT), and Positron Emission Tomography (PET). Publications relevant to the topic and written in English were selected. This literature review is based on 34 scientific articles published between 2005 and 2023.
Perineural tumor growth, involving the extension of tumor cells along nerves, is classified into Perineural Invasion (PNI) and Perineural Spread (PNS). PNI refers to neoplastic infiltration into small, unnamed peripheral nerve branches near the primary tumor site, typically identified histologically. In contrast, PNS involves tumor spread along larger, named nerves, often extending far from the primary tumor. Unlike PNI, PNS is usually detected through imaging modalities like MRI or CT and presents with symptoms related to the affected nerve [1].
According to statistics, 40% of patients with PNS exhibit no clinical symptoms, which makes it critically important to actively screen all patients with head and neck tumors for PNS, even in the absence of clinical signs of nerve damage [2]. This is very important because PNS leads to a more aggressive disease course, a higher risk of recurrence, and worse survival prognosis, thus emphasizing the need to optimize PNS evaluation. Proper diagnostic assessment of PNS requires not only the appropriate imaging techniques but also a thorough understanding of the commonly affected nerve pathways, tumor types associated with PNS, and characteristic radiological features.
Head and neck cancers encompass various malignancies, including those of the oral cavity, pharynx, larynx, paranasal sinuses, and salivary glands, as described in the NCCN Guidelines for Head and Neck Cancers [3]. Among these, the oral cavity is the most frequently affected site (37.3%), followed by the pharynx (16.2%), larynx (13.9%), nasal/paranasal sinuses (11.3%), neck (10.7%), and salivary glands (7.5%) [4]. Globally, head and neck cancers rank number seven in the list of types of most common cancers, with a higher prevalence in Southeast Asia and a lower incidence in developed countries [11]. In the U.S., head and neck cancers make up 4% of all cancers, with 71,110 new cases and 16,110 deaths expected in 2024 [7]. Incidence is higher in men, and it peaks in the 65–69 age group for men and 85–89 for women, with risk factors including tobacco use, alcohol consumption, and HPV infection [6–8].
Unlike many cancers, head and neck malignancies uniquely spread along nerves (PNS), occurring in 2.5%–5% of all cases [2]. Perineural Spread (PNS) is observed in various types of head and neck cancers, most notably, Adenoid Cystic Carcinoma (ACC), Squamous Cell Carcinoma (SCC), basal cell carcinoma, and melanoma [9]. Although ACC has the highest reported PNI rates (up to 56.4% (±19.0)), it is relatively rare, comprising only 1%–3% of head and neck cancers, and thus it contributes less frequently to PNS cases overall [10]. In contrast, SCC, which is the most common head and neck cancer (accounting for approximately 90% of the head and neck tumor cases), is responsible for the majority of PNS cases due to its prevalence. While the PNS rates of SCC are lower than those of ACC (14%–63.2%), they are significant, particularly in midface tumors, advanced stages, poorly differentiated subtypes, and recurrent disease [11,12]. Other tumors have a lower potential for perineural spread: basal cell carcinoma (0.2%–3%), mucoepidermoid carcinoma (29%), and desmoplastic melanoma (32%) [1]. These differences in the PNS rates may stem from variations in tumor biology as well as differences in study methodologies [11].
Perineural Spread (PNS) is a critical imaging feature in head and neck malignancies, as it impacts staging, treatment planning, risk assessment, and ultimately, the patient’s prognosis.
Radiological imaging plays a vital role in the treatment of patients with Perineural Spread (PNS). It is particularly important for assessing whether surgery is a viable option. For example, patients with tumor involvement in the basal cisterns are typically not considered for surgery due to the high risk of leptomeningeal dissemination and the challenges of accessing these deep-seated areas [13]. In addition, imaging helps define the extent of surgical resection. When PNS involves the intratemporal facial nerve in cases of malignant skin or parotid tumors, a mastoidectomy and removal of the facial nerve may be required along with tumor resection. However, if these nerve segments remain unaffected, such extensive procedures can be avoided [13]. However, surgical treatment alone is frequently insufficient for managing PNS, as most cases require a multidisciplinary approach that combines surgery with chemotherapy and/or radiotherapy. Imaging is indispensable not only for guiding these complex treatment strategies but also for evaluating their efficacy and detecting disease progression or recurrence [14].
For diagnosing, imaging often becomes the only tool in the head and neck region, as PNS often damages areas inaccessible for biopsy [13].
The prognostic value of perineural involvement depends on the tumor’s histologic subtype and its primary site. A meta-analysis by Tao et al. [15] of 74 studies involving 27,559 patients found that PNI significantly worsened the survival outcomes, including reduced overall survival (HR: 1.91, 95% CI: 1.71–2.13), disease-specific survival (HR: 1.79, 95% CI: 1.55–2.07), and disease-free survival (HR: 1.82, 95% CI: 1.69–1.96). PNI-positive patients also faced higher risks of local recurrence (HR: 2.54, 95% CI: 1.93–3.33), locoregional recurrence (HR: 2.27, 95% CI: 1.82–2.82), and distant metastasis (HR: 1.82, 95% CI: 1.34–2.48), with distant metastasis-free survival especially compromised (HR: 2.97, 95% CI: 1.82–4.85). PNI is also a negative prognostic feature in other head and neck malignancies. For instance, in mucoepidermoid carcinoma, PNI-positive patients have significantly lower 5-year disease-free survival rates compared to PNI-negative patients (57.7% vs. 88.8%). [16]. These findings confirm PNI as a negative prognostic marker across head and neck cancers, correlating with poorer local and regional control, increased metastasis, and reduced survival.
Imaging is a critical component in the evaluation and management of head and neck cancers, making it essential to employ the most effective imaging techniques. Commonly used modalities include CT, MRI, and PET.
Magnetic resonance imaging (MRI) is considered the most reliable technique for detecting perineural spread (PNS) due to its superior soft tissue contrast, multiplanar imaging capabilities, and accuracy in assessing nerve involvement [17]. These features are critical for identifying subtle nerve changes indicative of PNS. Additionally, MRI avoids ionizing radiation, which makes it safer for repeated imaging, particularly in younger or vulnerable populations.
A meta-analysis by Abdullaeva et al. [17], reviewing 11 retrospective studies, evaluated MRI’s diagnostic accuracy for PNS in head and neck tumors, by using histopathology or surgery as the gold standard. The pooled sensitivity was 0.85 (0.70–0.95), specificity 0.85 (0.80–0.89), a Positive Predictive Value (PPV) of 0.86 (0.70–0.94), and a Negative Predictive Value (NPV) of 0.85 (0.71–0.93). Significant heterogeneity was observed in sensitivity (I² = 72%, p = 0.003) and PPV (I² = 70%, p = 0.038), but not in specificity (I² = 12%, p = 0.842) or NPV (I² = 65%, p = 0.119). However, the sensitivity of MRI in accurately depicting the complete anatomical distribution of PNS is relatively lower, ranging between 63% and 89% [18]. The heterogeneity in the findings of Abdullaeva et al.’s meta-analysis [17] may stem from variations in the MRI parameters, such as the slice thickness, magnetic field strength (1.5T vs. 3T), and differences in protocols, including the conventional MRI versus MRI neurography. Additionally, only three studies have so far employed consecutive sampling, introducing potential bias by excluding certain patients without clear evidence of PNS. Despite these limitations, the study underscores the high diagnostic value of MRI, which could further improve with advancements in radiological technology, such as higher field strength MRI devices and improved neuroimaging protocols. This highlights the role of MRI as the gold standard for PNS imaging for head and neck cancers.
Computed Tomography (CT) has notable limitations in imaging Perineural Spread (PNS), especially when compared to Magnetic Resonance Imaging (MRI). CT is less effective at visualizing the soft tissue contrast and smaller anatomical structures essential for evaluating nerve involvement. It typically provides only indirect signs of PNS, such as widening or erosion of skull base foramina. As a result, CT tends to detect PNS at later stages, when significant changes in both tissue and bone are already present [1]. Furthermore, CT struggles in regions prone to bone artifacts, including the skull base, foramina, and canals, due to beam hardening and partial volume effects. That is shown in a study conducted by Hanna et al. [19], which evaluated perineural spread of adenoid cystic carcinoma to the skull base, where it was found that MRI offers higher sensitivity and specificity than CT in detecting PNS in this region. Another drawback of CT is its susceptibility to metal artifacts which are caused by dental fillings or implants [20]. These artifacts can create streaks and further degrade the quality of the image, therefore complicating the assessment of the surrounding structures.
Despite these limitations, Computed Tomography (CT) offers several advantages in the evaluation of head and neck tumors. CT is particularly effective for assessing bony structures and detecting acute hemorrhages. Furthermore, CT plays a critical role in guiding biopsy procedures for these tumors and in the imaging of patients with various implants which are incompatible with MRI [2].
Limited data exist on the diagnostic performance of 18F-FDG PET/CT for detecting Perineural Spread (PNS) in head and neck cancers, and its relatively infrequent use in this context is attributable to several factors. First, interpreting physicians may lack familiarity with the characteristic imaging findings of PNS, or else they may fail to consider it as a diagnostic possibility [1]. Additionally, PET/MRI studies are prone to false positives in cases of inflammatory or infectious processes, such as reactive lymph nodes or post-radiochemotherapy changes, which can mimic malignancy [21]. The relatively lower spatial resolution of 18F-FDG PET also presents challenges, as small-volume lesions characteristic of PNS in cranial nerves may go undetected, particularly when these lesions exhibit a limited FDG uptake [22]. Finally, physiologic brain uptake, especially at the skull base, can obscure subtle findings and complicate the interpretation of PET images [22].
Despite its limitations, many researchers emphasize that 18F-FDG PET provides valuable functional and metabolic insights for evaluating head and neck tumors and Perineural Spread (PNS), particularly when used in conjunction with the conventional imaging modalities. Several studies [1,23] highlight that PET and the traditional imaging techniques, such as MRI and CT, should be considered complementary in assessing PNS. The combined use of PET’s metabolic data and the structural information from MRI and CT offers a more accurate depiction of the full extent of PNS, which may be challenging to achieve with MRI alone. Moreover, as noted in a study of Park et al. [21], PET/MRI demonstrates superior diagnostic performance in both accuracy and confidence compared to PET or MRI used independently, further supporting the integrative approach for head and neck cancer evaluation. Beyond PNS evaluation, 18F-FDG PET/CT offers significant benefits in the management of head and neck tumors. For example, PET/CT detects cervical lymph node metastases with higher sensitivity and accuracy than MRI [24]. Additionally, the multi-modal integration of co-registered PET/CT and MRI provides superior results for assessing trans-compartmental extensions in T and N staging compared to using individual techniques [25]. Furthermore, PET/CT also plays a crucial role in detecting distant metastases and identifying occult primary tumors [26].
Another critical application of PET imaging has been the evaluation of tumor recurrence and Perineural Spread (PNS) following treatment. However, recent studies increasingly favor diffusion-weighted imaging (DWI) over PET for post-treatment assessment of head and neck tumors. For example, Schroeder et al. [24] reported that MRI demonstrated sensitivity, specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) of 62% (5/8), 88% (15/17), 71% (5/7), and 83% (15/18), respectively, for detecting perineural spread. In contrast, PET/CT failed to detect any cases of PNS. Furthermore, a significant drawback of PET/CT is its high false-positive rate, which is particularly problematic when scans are performed within 8–12 weeks after treatment, as treatment-related inflammation often confounds the results. This does not apply to DWI, and it can be performed earlier than 8 weeks after chemoradiotherapy, thus allowing for faster identification of tumor recurrence and treatment [27].
The radiological manifestations of Perineural Spread (PNS) comprise two distinct categories: primary findings, which encompass structural alterations of the nerve and its adjacent tissues, and secondary findings, which manifest as changes within the denervated territory supplied by the affected nerve.
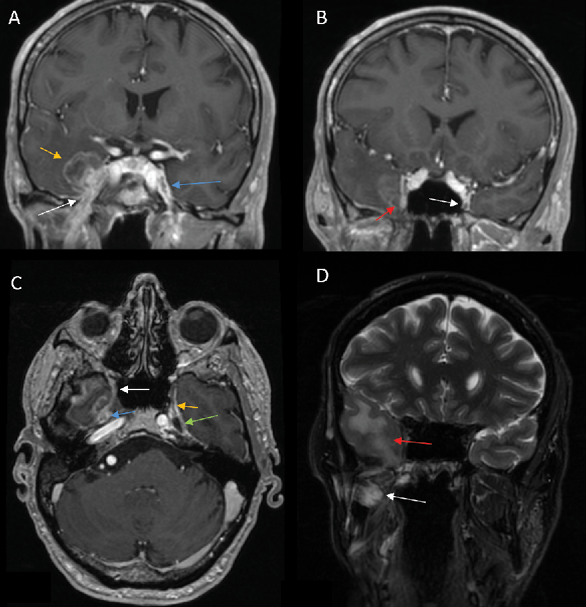
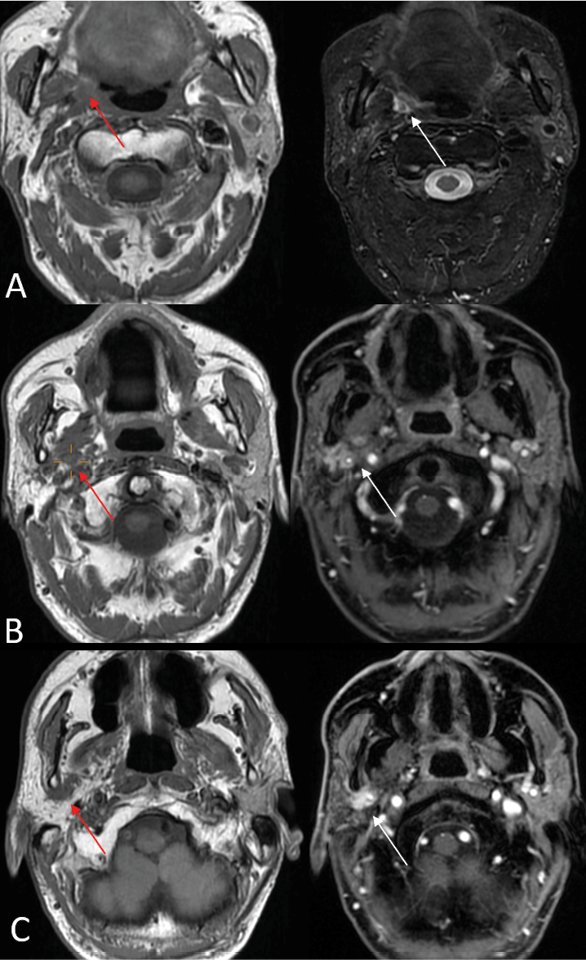
1) PNS commonly presents as enhancement of the affected nerve, which is attributed to the disruption of the blood-nerve barrier caused by the tumor growth and the associated nerve damage [28]. This enhancement is frequently accompanied by nerve enlargement due to multiple pathophysiological processes including tumor infiltration, inflammation, interstitial edema, and neurotrophic factor-induced hypertrophy [1]. However, in some instances, enhancement may occur without appreciable nerve enlargement, thus creating diagnostic challenges in radiological detection. Magnetic Resonance Imaging (MRI) optimally demonstrates both neural enhancement and enlargement, particularly when extending through neural foramina. Pathologic enhancement is characterized by diffuse, uninterrupted enhancement with no clear demarcation from the Perineural Vascular Plexus (PNVP) (Fig. 1, A, B and C) [2]. Careful evaluation of the enhancement intensity, thickness, and symmetry between the sides is essential to identify pathological changes. On CT, although individual nerves may not be well-defined, excessive contrast enhancement in neural foramina or canals can indicate nerve involvement [29].
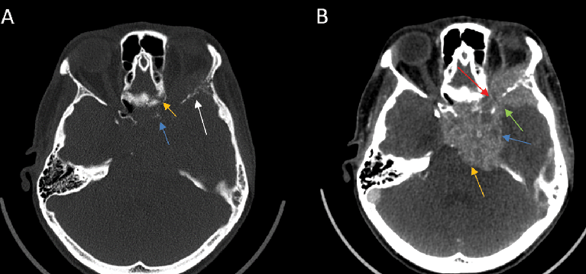
2) Enlargement and/or Erosion of Neural Foramina or Canals (Figs. 2–3). Perineural Spread (PNS) can manifest radiologically as morphological alterations of neural passageways, characterized by foraminal or canal enlargement secondary to neural expansion. This is a delayed finding, as the normal nerve is smaller than the foramen, and it has ample time to enlarge before bone destruction becomes evident. On imaging studies, this enlargement of foramina typically presents as bilateral asymmetry. However, isolated foraminal enlargement demonstrates limited specificity, as it may represent benign pathological processes or anatomical variants. The presence of concurrent foraminal or canal erosive changes provides stronger radiological evidence for malignant processes, particularly PNS, thereby carrying greater diagnostic significance [29]. Enlargement or erosion of neural foramina or canals is more effectively visualized on CT compared to MRI.
3) Obliteration of Fat Planes and pterygopalatine fossa (Fig. 4 C). In Perineural Spread (PNS), tumor invasion can disrupt and replace the normal fat planes surrounding nerves, which serve as a natural barrier visible on imaging. This obliteration, often seen as a loss of the normal hypodense fat signal on CT or the hyperintense fat signal on MRI T1 sequences, indicates tumor infiltration or extension, aiding in the diagnosis of PNS [2].

4) On CT or MR imaging, replacement of the fluid signal in Meckel’s cave by a solid and enhancing lesion (Fig 1 C) indicates invasion of the trigeminal cistern [29].
5) On CT and MRI, cavernous sinus invasion is characterized by enlargement of the cavernous sinus, with lateral bulging and increased convexity. However, on CT, it can be challenging to distinguish the enhancing tumor tissue from the venous-filled cavernous sinus on T1-enhanced sequences. [30].
6) The primary imaging feature of PNS on 18F-FDG PET/CT is linear or curvilinear increased 18F-FDG uptake along the distribution of the CN relative to activity in the surrounding tissue, which may be continuous or discontinuous with the primary tumor [1].
1) Denervation of muscles
On MRI, denervated muscles undergo a characteristic pattern of change:
In the acute phase (<1 month), MRI findings are characterized by T2 hyperintense edema-like signals, best visualized on fat-suppressed T2-weighted sequences (Fig. 1 D), and contrast enhancement of the affected muscles, which is most prominent on post-contrast, fat-suppressed T1-weighted images. An increased muscle volume is also noted [29]. During the first four weeks of denervation, there is a shift in water distribution, with a relative decrease in intracellular water and a corresponding increase in extracellular water, while the total tissue water remains unchanged. This redistribution leads to hyperintense signals on T2-weighted MR images, mimicking edema, as the T2 relaxation time of extracellular water is longer than that of intracellular water [31]. Additionally, enhanced contrast uptake is observed in the affected muscles due to increased perfusion and contrast medium accumulation in the extracellular space [31]. Notably, the denervated muscle retains its internal striation, distinguishing it from the muscle infiltrated by tumors, which disrupts the muscular architecture [2]. On PET imaging, there is typically increased 18F-FDG uptake in the affected musculature due to hypermetabolism associated with acute denervation [32].
In the subacute phase (up to 12–20 months), there is a progression from edema-like changes to fatty transformation and chronic modifications in the muscles. While muscles may still exhibit T2 prolongation and contrast enhancement, there is neither an increase nor a loss in the muscle volume. Additionally, T1 hyperintensity becomes apparent, reflecting fat infiltration [33]. In the subacute phase of denervation, PET imaging typically shows normalized or slightly increased 18F-FDG uptake in the affected musculature [32].
The chronic phase of denervation (12–20 months post-onset) is characterized by diffuse fatty infiltration of muscles, which appears as hyperintensity on both T1- and T2-weighted MRI images, along with a reduction in the muscle volume, detectable on both MRI and computed tomography (CT) [33] . Denervation atrophy is most clearly visualized on T1- and T2-weighted images without fat suppression, as the use of fat suppression techniques can obscure the appearance of fatty muscle replacement [29]. In contrast, direct neoplastic muscle infiltration typically results in an increased muscle volume and more heterogeneous signal alterations, with a lower signal intensity compared to the hyperintense changes seen in chronic denervation atrophy [2]. In addition, in this phase on PET imaging, there is muscle atrophy with a decreased 18F-FDG uptake in the affected muscles, along with an increased compensatory uptake in contralateral unaffected muscles, such as the contralateral tongue in CN XII or vocal in CN X cord denervation [1].
2) Thickening and/or enhancement of SMAS
SMAS is an organized fibrous network which connects the facial muscles, which originate on bones, to the dermis, amplifying the effect of muscle movement on the skin. It divides the subcutaneous fat into two layers: a superficial layer with small fat lobules enclosed by fibrous septa extending toward the dermis, and a deeper layer containing larger, unpartitioned fat deposits. The peripheral branches of the facial nerve (CN VII) — temporal, zygomatic, buccal, marginal mandibular, and cervical — exit the parotid gland and travel deep to SMAS before innervating facial expression muscles, whereas sensory branches of the trigeminal nerve lie superficial to SMAS [34]. On CT, SMAS appears as a hyperattenuating arcuate line, whereas, on MRI, it is hypointense on both T1 and T2 sequences, visible within subcutaneous fat beneath the skin and above the facial muscles without a distinct origin or insertion [34]. Imaging defines SMAS as restricted to the facial region, blending with the parotid and temporoparietal fascia. Nodular thickening and enhancement of SMAS structures on imaging can serve as secondary features indicating Perineural Spread (PNS) along peripheral CN V or CN VII branches [29]. This subtle yet significant radiological finding in the Perineural Spread (PNS) is frequently underreported in diagnostic imaging [14].
3) Eustachian tube dysfunction
Ipsilateral findings of torus tubarius hypoplasia, mastoid opacification, or middle ear effusion may indicate mandibular nerve (V3) denervation of the tensor veli palatini muscle, resulting in eustachian tube dysfunction [2].
A summary of all the imaging features of PNS is provided in Table 1.
|
Category |
Feature |
Description |
Best imaging modality to detect changes |
|
Primary Imaging Features |
Nerve enhancement/enlargement |
– |
MRI (T1 post-contrast) |
|
Enlargement/erosion of neural foramina or canals |
– |
CT |
|
|
Obliteration of fat planes and pterygopalatine fossa |
Loss of the normal hyperintense signal of fat surrounding the nerve, replaced by a hypointense signal corresponding to soft tissue (tumor infiltration). |
MRI (T1) |
|
|
Meckel’s cave invasion |
The normal hypointense cerebrospinal fluid (CSF) signal in Meckel’s cave is replaced by a hyperintense signal consistent with soft tissue (indicative of tumor infiltration). |
MRI (T2) |
|
|
Enlargement of the cavernous sinus |
Structural distortion and bulging of the cavernous sinus with irregular or nodular enhancement replacing normal homogeneous enhancement. |
MRI (T1 post-contrast) |
|
|
Linear or curvilinear increased 18F-FDG uptake |
– |
PET |
|
|
Secondary Imaging Features |
Muscle denervation |
||
|
Acute (<1 month) |
Edema-like T2 hyperintensity, contrast enhancement (CE), muscle volume increase, and increased 18F-FDG uptake on PET. |
MRI (T2 with fat suppression, T1 post-contrast), PET |
|
|
Subacute (up to 12–20 months) |
T2 hyperintensity, T1 hyperintensity, contrast enhancement (CE), normal muscle volume, and normalized or slightly increased 18F-FDG uptake on PET. |
MRI (T1, T2 with fat suppression, T1 post-contrast), PET |
|
|
Chronic (>12–20) |
T2 hyperintensity, T1 hyperintensity, no contrast enhancement (CE), muscle volume decrease, decreased 18F-FDG uptake, and increased uptake in contralateral muscles on PET. |
MRI (T1, T2), PET |
|
|
Thickening and/or enhancement of SMAS. |
– |
MRI (T1 post-contrast) |
|
|
Eustachian tube dysfunction (m.tensor veli palatini denervation) |
Ipsilateral findings of torus tubarius hypoplasia, mastoid opacification, or middle ear effusion |
MRT |
|
Nearly all of the imaging findings mentioned above are not exclusive to PNS and could be associated with other diseases. In most cases, these imaging findings serve as key indicators of a potential pathological condition, often in conjunction with the patient’s history and clinical symptoms, which ultimately help in reaching the correct diagnosis.
1) Head and neck tumors represent a heterogeneous group of neoplasms and rank seventh in prevalence among different tumor types. These tumors have a propensity for Perineural Spread (PNS), with Adenoid Cystic Carcinoma (ACC) and Squamous Cell Carcinoma (SCC) being the histological subtypes most commonly associated with PNS.
2) Radiological imaging plays a crucial role in the diagnosis of PNS and treatment planning, as its detection significantly impacts patient prognosis.
3) Due to its superior soft tissue resolution, MRI is considered the gold standard for PNS diagnosis, with an estimated sensitivity and specificity of approximately 85%.
4) CT imaging is particularly useful for visualizing bony involvement, and it plays an important role in guiding biopsies.
5) Combined PET imaging provides a more accurate assessment of the PNS extent and lymph node involvement.
6) For evaluating tumor recurrence and PNS after treatment, MRI with Diffusion-Weighted Imaging (DWI) is preferred over PET.
7) Most important primary PNS features are as follows: nerve enhancement/enlargement, enlargement/erosion of neural foramina or canals, obliteration of fat planes and pterygopalatine fossa, Meckel’s cave invasion, enlargement of the cavernous sinus, and linear or curvilinear increased 18F-FDG uptake.
8) Most important secondary PNS features are as follows: muscle denervation (acute, subacute and chronic stages), thickening and/or enhancement of SMAS, eustachian tube dysfunction (m.tensor veli palatini denervation).
9) While most imaging findings are not exclusive to PNS, their presence, when correlated with clinical history and symptoms, serves as a crucial diagnostic indicator, guiding accurate identification of the condition.